Identification of a stress-responsive subregion of the basolateral amygdala in male rats
- PMID: 39117904
- PMCID: PMC11480132
- DOI: 10.1038/s41386-024-01927-x
Identification of a stress-responsive subregion of the basolateral amygdala in male rats
Abstract
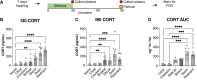
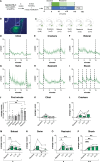
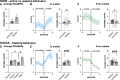
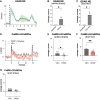
The basolateral amygdala (BLA) is reliably activated by psychological stress and hyperactive in conditions of pathological stress or trauma; however, subsets of BLA neurons are also readily activated by rewarding stimuli and can suppress fear and avoidance behaviours. The BLA is highly heterogeneous anatomically, exhibiting continuous molecular and connectivity gradients throughout the entire structure. A critical gap remains in understanding the anatomical specificity of amygdala subregions, circuits, and cell types explicitly activated by acute stress and how they are dynamically activated throughout stimulus exposure. Using a combination of topographical mapping for the activity-responsive protein FOS and fiber photometry to measure calcium transients in real-time, we sought to characterize the spatial and temporal patterns of BLA activation in response to a range of novel stressors (shock, swim, restraint, predator odour) and non-aversive, but novel stimuli (crackers, citral odour). We report four main findings: (1) the BLA exhibits clear spatial activation gradients in response to novel stimuli throughout the medial-lateral and dorsal-ventral axes, with aversive stimuli strongly biasing activation towards medial aspects of the BLA; (2) novel stimuli elicit distinct temporal activation patterns, with stressful stimuli exhibiting particularly enhanced or prolonged temporal activation patterns; (3) changes in BLA activity are associated with changes in behavioural state; and (4) norepinephrine enhances stress-induced activation of BLA neurons via the ß-noradrenergic receptor. Moving forward, it will be imperative to combine our understanding of activation gradients with molecular and circuit-specificity.
© 2024. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
MN Hill is an Associate Editor at
Figures

References
-
- McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N. Y Acad Sci. 1998;840:33–44. 10.1111/j.1749-6632.1998.tb09546.x - PubMed
-
- Ipser JC, Singh L, Stein DJ. Meta-analysis of functional brain imaging in specific phobia: Imaging meta-analysis of specific phobia. Psychiatry Clin Neurosci. 2013;67:311–22. 10.1111/pcn.12055 - PubMed
-
- Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J Comp Neurol. 2008;508:458–72. 10.1002/cne.21687 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

