Hypermethylated RASAL1's promotive role in chemoresistance and tumorigenesis of choriocarcinoma was regulated by TET2 but not DNMTs
- PMID: 39118077
- PMCID: PMC11312928
- DOI: 10.1186/s12885-024-12758-w
Hypermethylated RASAL1's promotive role in chemoresistance and tumorigenesis of choriocarcinoma was regulated by TET2 but not DNMTs
Abstract
Background: Patients with choriocarcinoma (CC) accompanying chemoresistance conventionally present a poor prognosis. Whether ras protein activator like-1 (RASAL1) functions as a tumor promoter or suppressor depends on tumor types. However, the role of RASAL1 in process of chemoresistance of CC and underlying molecular mechanism remain elusive.
Methods: The expression pattern of RASAL1 in CC cells and tissues was measured using Western blotting, immunohistochemistry and qRT-PCR. Cell viability and proliferative ability were assessed by MTT assay, Tunnel assay and flow cytometric analysis. Additionally, the stemness was evaluated by the colony formation and tumor sphere formation. Methotrexate (MTX) was applied to exam the chemosensitivity of CC cells.
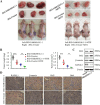
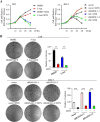
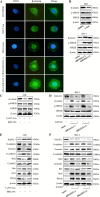
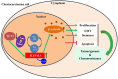
Results: The expression of RASAL1 was reduced both at the protein and mRNA levels in CC tissues and cells compared to hydatidiform mole (HM) and invasive mole (IM). Loss of RASAL1 was attributed to its promoter hypermethylation and could be restored by 5-Aza. Knock-down of RASAL1 promoted the viability, proliferative potential, stemness and EMT phenotype of JEG-3 cells. However, induced overexpression of RASAL1 by 5-Aza significantly prohibited cell proliferation and stemness potential of the JAR cell. Additionally, the xenograft model indicated that knockdown of RASAL1 led to a remarkable increase of tumor volume and weight in comparison with its counterpart. Moreover, the stimulatory activity brought by decrease of RASAL1 could be deprived by β-catenin inhibitor XAV 939, yet the suppressive activity resulted from its promoter demethylation could be rescued by β-catenin activator BML-284, indicating that function of RASAL1 depends on β-catenin. Besides, the co-immunoprecipitation assay confirmed the physical binding between RASAL1 and β-catenin. Further investigations showed hypermethylated RASAL1 was regulated by TET2 but not DNMTs.
Conclusion: Taken together, the present data elucidated that reduced RASAL1 through its promoter hypermethylation regulated by TET2 promoted the tumorigenicity and chemoresistance of CC via modulating β-catenin both in vitro and in vivo.
Keywords: Chemoresistance; Choriocarcinoma; RASAL1; TET2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Secreted Frizzled Related Protein 2 Modulates Epithelial-Mesenchymal Transition and Stemness via Wnt/β-Catenin Signaling in Choriocarcinoma.Cell Physiol Biochem. 2018;50(5):1815-1831. doi: 10.1159/000494862. Epub 2018 Nov 5. Cell Physiol Biochem. 2018. PMID: 30396168
-
[Changes in gene expression profiles of hydatidiform mole and choriocarcinoma as compared with trophoblast hyperplasia].Zhonghua Zhong Liu Za Zhi. 2004 Dec;26(12):727-31. Zhonghua Zhong Liu Za Zhi. 2004. PMID: 15733390 Chinese.
-
miR-21 Is Overexpressed in Hydatidiform Mole Tissues and Promotes Proliferation, Migration, and Invasion in Choriocarcinoma Cells.Int J Gynecol Cancer. 2017 Feb;27(2):364-374. doi: 10.1097/IGC.0000000000000861. Int J Gynecol Cancer. 2017. PMID: 27922982
-
Hypermethylation and loss of retinoic acid receptor responder 1 expression in human choriocarcinoma.J Exp Clin Cancer Res. 2017 Nov 23;36(1):165. doi: 10.1186/s13046-017-0634-x. J Exp Clin Cancer Res. 2017. PMID: 29169400 Free PMC article.
-
Hypermethylation and clinicopathological significance of RASAL1 gene in gastric cancer.Asian Pac J Cancer Prev. 2013;14(11):6261-5. doi: 10.7314/apjcp.2013.14.11.6261. Asian Pac J Cancer Prev. 2013. PMID: 24377515
References
-
- Kaur B. Pathology of gestational trophoblastic disease (GTD). Best practice & research Clinical obstetrics & gynaecology 2021, 74:3–28. - PubMed
-
- Abu-Rustum NR, Yashar CM, Bean S, Bradley K, Campos SM, Chon HS, Chu C, Cohn D, Crispens MA, Damast S, et al. Gestational trophoblastic neoplasia, Version 2.2019, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Cancer Network: JNCCN. 2019;17(11):1374–91. 10.6004/jnccn.2019.0053 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

