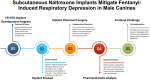
Bioabsorbable, subcutaneous naltrexone implants mitigate fentanyl-induced respiratory depression at 3 months-A pilot study in male canines
- PMID: 39118319
- PMCID: PMC11310269
- DOI: 10.14814/phy2.16176
Bioabsorbable, subcutaneous naltrexone implants mitigate fentanyl-induced respiratory depression at 3 months-A pilot study in male canines
Abstract
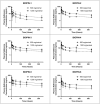
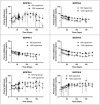
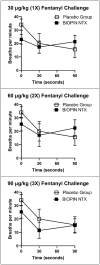
The aim of this study is to determine if extended-release, bioabsorbable, subcutaneous naltrexone (NTX) implants can mitigate respiratory depression after an intravenous injection (IV) of fentanyl. Six different BIOabsorbable Polymeric Implant Naltrexone (BIOPIN) formulations, comprising combinations of Poly-d,l-Lactic Acid (PDLLA) and/or Polycaprolactone (PCL-1 or PCL-2), were used to create subcutaneous implants. Both placebo and naltrexone implants were implanted subcutaneously in male dogs. The active naltrexone implants consisted of two doses, 644 mg and 1288 mg. A challenge with IV fentanyl was performed in 33 male dogs at 97-100 days after implantation. Following the administration of a 30 μg/kg intravenous fentanyl dose, the placebo cohort manifested a swift and profound respiratory depression with a ~50% reduction in their pre-dose respiratory rate (RR). The BIOPIN NTX-implanted dogs were exposed to escalating doses of intravenous fentanyl (30 μg/kg, 60 μg/kg, 90 μg/kg, and 120 μg/kg). In contrast, the dogs implanted with the BIOPIN naltrexone implants tolerated doses up to 60 μg/kg without significant respiratory depression (<50%) but had severe respiratory depression with fentanyl doses of 90 μg/kg and especially at 120 μg/kg. Bioabsorbable, extended-release BIOPIN naltrexone implants are effective in mitigating fentanyl-induced respiratory depression in male canines at about 3 months after implantation. This technology may also have potential for mitigating fentanyl-induced respiratory depression in humans.
Keywords: bioabsorbable; canines; fentanyl; implants; naltrexone; respiratory depression.
© 2024 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
JDB and SMC have a financial interest in The Drug Delivery Company, LLC, which is the licensee of USPTO# 11,197,819 B1. MF has been a consultant for The Drug Delivery Company LLC. JAH, RLJ, DD, and VH have no relevant financial disclosures.
Figures



References
-
- Bartus, R. T. , Emerich, D. F. , Hotz, J. , Blaustein, M. , Dean, R. L. , Perdomo, B. , & Basile, A. S. (2003). Vivitrex, an injectable, extended‐release formulation of naltrexone, provides pharmacokinetic and pharmacodynamic evidence of efficacy for 1 month in rats. Neuropsychopharmacology, 28(11), 1973–1982. 10.1038/sj.npp.1300274 - DOI - PubMed
-
- Benner, J. D. , Cohen, S. M. , Vutukuru, N. K. R. , Kulkarni, P. S. , & Shanmugam, S. (2021). Extended release bioabsorbable subcutaneous medicinal dosage delivery implant system. https://pubchem.ncbi.nlm.nih.gov/patent/US‐11197819‐B1
-
- ClinicalTrials.gov . (2022). The O'Neil long acting naltrexone implant (OLANI) pharmacokinetic (PK)/safety study in healthy volunteers. https://clinicaltrials.gov/study/NCT03810495
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

