Endogenous RBM4 prevents Ang II-induced cardiomyocyte hypertrophy via downregulating the expression of PTBP1
- PMID: 39118568
- PMCID: PMC11986438
- DOI: 10.3724/abbs.2024103
Endogenous RBM4 prevents Ang II-induced cardiomyocyte hypertrophy via downregulating the expression of PTBP1
Abstract
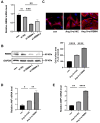
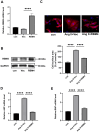
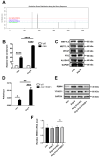
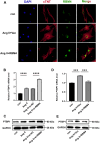
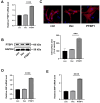
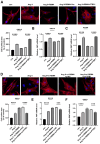
Aberrant gene expression in cardiomyocyte has been revealed to be the fundamental essence of pathological cardiac hypertrophy. However, the detailed mechanisms are not fully understood. The underlying regulators of gene expression involved in cardiac hypertrophy remain to be further identified. Here, we report that the RNA-binding protein RNA-binding motif protein 4 (RBM4) functions as an endogenic protector that is able to fight against cardiomyocyte hypertrophy in vitro. Under pro-hypertrophic stimulation of angiotensin II (Ang II), the protein level of RBM4 in cardiomyocyte and myocardium is elevated. Knockdown of RBM4 can further aggravate cardiomyocyte hypertrophy, while over-expression of RBM4 represses cardiomyocyte hypertrophy. Mechanistically, RBM4 is localized in the nucleus and down-regulates the expression of polypyrimidine tract-binding protein 1 (PTBP1), which has been shown to aggravate cardiomyocyte hypertrophy. In addition, we suggest that the up-regulation of RBM4 in cardiomyocyte hypertrophy is caused by N6-methyladenosine (m6A). Ang II induces m6A methylation of RBM4 mRNA, which further enhances the YTH domain-containing family protein 1 (YTHDF1)-mediated translation of RBM4. Thus, our results reveal a novel pathway consisting of m6A, RBM4 and PTBP1, which is involved in cardiomyocyte hypertrophy.
Keywords: PTBP1; RBM4; cardiomyocyte hypertrophy; m6A.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. . 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. - DOI - PubMed
-
- Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. . 2016;97:245–262. doi: 10.1016/j.yjmcc.2016.06.001. - DOI - PubMed
-
- Li Z, Guo Q, Zhang J, Fu Z, Wang Y, Wang T, Tang J. The RNA-binding motif protein family in cancer: friend or foe? Front Oncol. . 2021;11:757135. doi: 10.3389/fonc.2021.757135. - DOI - PMC - PubMed
-
- van den Hoogenhof MMG, Beqqali A, Amin AS, van der Made I, Aufiero S, Khan MAF, Schumacher CA, et al. RBM20 mutations induce an arrhythmogenic dilated cardiomyopathy related to disturbed calcium handling. Circulation. . 2018;138:1330–1342. doi: 10.1161/CIRCULATIONAHA.117.031947. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

