Main chain selective polymer degradation: controlled by the wavelength and assembly
- PMID: 39118612
- PMCID: PMC11304539
- DOI: 10.1039/d4sc02172j
Main chain selective polymer degradation: controlled by the wavelength and assembly
Abstract
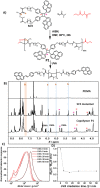
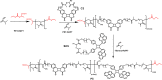
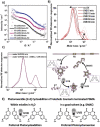
The advent of reversible deactivation radical polymerization (RDRP) revolutionized polymer chemistry and paved the way for accessing synthetic polymers with controlled sequences based on vinylic monomers. An inherent limitation of vinylic polymers stems from their all-carbon backbone, which limits both function and degradability. Herein, we report a synthetic strategy utilizing radical ring-opening polymerization (rROP) of complementary photoreactive cyclic monomers in combination with RDRP to embed photoresponsive functionality into desired blocks of polyvinyl polymers. Exploiting different absorbances of photoreactive cyclic monomers, it becomes possible to degrade blocks selectively by irradiation with either UVB or UVA light. Translating such primary structures of polymer sequences into higher order assemblies, the hydrophobicity of the photodegradable monomers allowed for the formation of micelles in water. Upon exposure to light, the nondegradable blocks detached yielding a significant reduction in the micelle hydrodynamic diameter. As a result of the self-assembled micellar environment, telechelic oligomers with photoreactive termini (e.g., coumarin or styrylpyrene) resulting from the photodegradation of polymers in water underwent intermolecular photocycloaddition to photopolymerize, which usually only occurs efficiently at longer wavelengths and a much higher concentration of photoresponsive groups. The reported main chain polymer degradation is thus controlled by the irradiation wavelength and the assembly of the polymers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

