Shallow conductance decay along the heme array of a single tetraheme protein wire
- PMID: 39118640
- PMCID: PMC11304805
- DOI: 10.1039/d4sc01366b
Shallow conductance decay along the heme array of a single tetraheme protein wire
Abstract
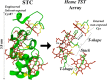
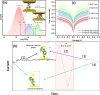
Multiheme cytochromes (MHCs) are the building blocks of highly conductive micrometre-long supramolecular wires found in so-called electrical bacteria. Recent studies have revealed that these proteins possess a long supramolecular array of closely packed heme cofactors along the main molecular axis alternating between perpendicular and stacking configurations (TST = T-shaped, stacked, T-shaped). While TST arrays have been identified as the likely electron conduit, the mechanisms of outstanding long-range charge transport observed in these structures remain unknown. Here we study charge transport on individual small tetraheme cytochromes (STCs) containing a single TST heme array. Individual STCs are trapped in a controllable nanoscale tunnelling gap. By modulating the tunnelling gap separation, we are able to selectively probe four different electron pathways involving 1, 2, 3 and 4 heme cofactors, respectively, leading to the determination of the electron tunnelling decay constant along the TST heme motif. Conductance calculations of selected single-STC junctions are in excellent agreement with experiments and suggest a mechanism of electron tunnelling with shallow length decay constant through an individual STC. These results demonstrate that an individual TST motif supporting electron tunnelling might contribute to a tunnelling-assisted charge transport diffusion mechanism in larger TST associations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Adhikari R. Y. Malvankar N. S. Tuominen M. T. Lovley D. R. RSC Adv. 2016;6:8354–8357. doi: 10.1039/C5RA28092C. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

