Timing of stereotactic radiosurgery within the first-line systemic treatment in non-small cell lung cancer brain metastases: a retrospective single-center cohort study
- PMID: 39118877
- PMCID: PMC11304149
- DOI: 10.21037/tlcr-24-132
Timing of stereotactic radiosurgery within the first-line systemic treatment in non-small cell lung cancer brain metastases: a retrospective single-center cohort study
Abstract
Background: Stereotactic radiosurgery/radiotherapy (SRS/SRT) and novel systemic treatments, such as tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs), have demonstrated to be effective in managing brain metastases in non-small cell lung cancer (NSCLC). However, the optimal treatment sequence of SRS/SRT and TKI/ICI remains uncertain. This retrospective monocentric analysis addresses this question by comparing the outcomes of patients with NSCLC brain metastases who received upfront SRS/SRT versus those who were initially treated with TKI/ICI.
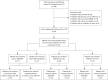
Methods: All patients treated with SRS/SRT and TKI/ICI for NSCLC brain metastases were collected from a clinical database. The patients who received first-line TKI or ICI for the treatment of brain metastases were then selected for further analysis. Within this cohort, a comparative analysis between upfront SRS/SRT and patients initially treated with TKI/ICI was conducted, assessing key parameters such as overall survival (OS), intracranial progression-free survival (iPFS) and treatment-related toxicity. Both OS and iPFS were defined as the time from SRS/SRT to either death or disease progression, respectively.
Results: The analysis encompassed 54 patients, of which 34 (63.0%) patients received SRS/SRT and TKI/ICI as their first-line therapy. Of the latter, 17 (50.0%) patients received upfront SRS/SRT and 17 (50.0%) were initially treated with TKI/ICI; 24 (70.6%) received SRS/SRT and ICI, and 10 (29.4%) received SRS/SRT and TKI. The cohorts did not significantly differ in the univariable analyses for the following parameters: sex, age, histology, molecular genetics, disease stage at study treatment, performance status, number of brain metastases, treatment technique, tumor volume, target volume, disease progression, radiation necrosis, dosimetry. While no significant differences were found in terms of iPFS and OS between patients treated with upfront SRS/SRT and patients initially treated with TKI, upfront SRS/SRT demonstrated significantly superior OS when compared to patients initially treated with ICI (median OS not reached vs. 17.5 months; mean 37.8 vs. 23.6 months; P=0.03) with no difference in iPFS. No significant differences in treatment-related toxicity were observed among the cohorts.
Conclusions: In this retrospective, single-center cohort study, patients treated with upfront SRS/SRT demonstrated significantly longer OS compared to patients initially treated with ICI in the cohort receiving first-line therapy for brain metastases. However, given the retrospective design and the limited cohort size, definitive conclusions cannot be drawn from these findings. Nevertheless, the results suggest that the timing of SRS/SRT may play an important role in treatment outcomes. Further investigation, preferably through prospective randomized trials, is warranted to provide more conclusive answers to this important question.
Keywords: Checkpoint inhibitors; immunotherapy; stereotactic radiosurgery (SRS); targeted treatment; tyrosine kinase inhibitors (TKIs).
2024 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-132/coif). R.B. received Open Access Fund from University of Tübingen, and received honoraria from NovoCure for participating in invited meetings of specialized centers. C.B. received grants or contracts not related to this manuscript from Viewray, Brainlab and Elekta, and received support for attending meetings and/or travel from BMS, Roche, Merck, AstraZeneca and Viewray. F.M. received an unrestricted Research Institutional Grant from AstraZeneca, received honoraria from AstraZeneca, Novartis, Roche, Lilly, Elekta and Brainlab, and serves in the advisory board of AstraZeneca and Novartis. M.N. received payments from Brainlab and AstraZeneca for speaking services. The other authors have no conflicts of interest to declare.
Figures

References
-
- Solomon BJ, Bauer TM, Mok TSK, et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med 2023;11:354-66. 10.1016/S2213-2600(22)00437-4 - DOI - PubMed
LinkOut - more resources
Full Text Sources
