Efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer patients with rare KRAS mutations: a real-world retrospective study
- PMID: 39118889
- PMCID: PMC11304142
- DOI: 10.21037/tlcr-24-372
Efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer patients with rare KRAS mutations: a real-world retrospective study
Abstract
Background: Kirsten rat sarcoma homolog (KRAS) mutations are one of the key drivers in non-small cell lung cancer (NSCLC) and FDA-approved specific inhibitors of KRAS-G12C mutation are available clinically. However, inhibitors of certain KRAS mutation subtypes remain unavailable, especially rare KRAS mutations including G13C, G13D, and Q61H. In this study, we retrospectively investigated the outcomes of NSCLC patients with rare KRAS-mutation to determine if they may benefit from immune checkpoint inhibitors (ICIs).
Methods: Our retrospective study involved 240 advanced NSCLC patients with KRAS mutations, who visited Shanghai Chest Hospital from July 2018 to July 2021. Complete clinical and pathological data were recorded and progression-free survival (PFS) and overall survival (OS) were adopted as primary endpoints.
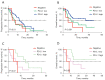
Results: The median follow-up time was 36.5 months (range, 30.8-42.1 months) and the median OS was 9.7 months (range, 7.6-11.8 months). Of the 240 patients evaluated, 130 (54.2%) received chemotherapy and 110 (45.8%) received ICI-based treatment. Among the patients who received chemotherapy, patients with rare KRAS-mutations presented worse survival outcomes (median PFS, 3.4 vs. 4.1 months, P=0.047; median OS, 5.2 vs. 7.1 months, P=0.02) than conventional KRAS-mutant patients. PFS and OS of rare KRAS-mutation patients were prolonged after immunotherapy (median PFS 7.3 vs. 3.4 months, P<0.001; median OS, 13.3 vs. 5.2 months, P<0.001) and had no significant difference compared with conventional KRAS-mutant patients, in part of them whose programmed death-ligand 1 (PD-L1) expression data before immunotherapy were available (n=72), patients with a higher rate of PD-L1 positive tumor cells (≥50%) presented elevated PFS and OS.
Conclusions: Despite having potential survival disadvantage compared with other NSCLC patients, rare KRAS-mutant patients (other than G12A, C, D, V) could benefit specifically from ICI-based therapy and survival outcomes are correlated with PD-L1 expression.
Keywords: Advanced non-small cell lung cancer (advanced NSCLC); Kirsten rat sarcoma homolog mutations (KRAS mutations); immune checkpoint inhibitor (ICI); rare KRAS mutations; survival benefit.
2024 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-372/coif). H.H. received grants or contracts from BMS, Lilly Oncology, Roche Diagnostics, BillionToOne; consulting fees from Mirati, Janssen, Astrazeneca, Regeneron, BMS, Guardant, Foundation Medicine, Merck, Regeneron; payment or honoraria from Amgen, Mirati, Foundation Medicine, Janssen, Astrazeneca, EMD Serono, Merck, Regeneron. F.F. serves as the Advisory board for MSD laboratory. The other authors have no conflicts of interest to declare.
Figures




Similar articles
-
PD-1/L1 immune checkpoint inhibitors for KRAS-mutant non-small cell lung cancer: a multicenter retrospective real-world study.BMC Cancer. 2025 Mar 12;25(1):444. doi: 10.1186/s12885-025-13868-9. BMC Cancer. 2025. PMID: 40075326 Free PMC article.
-
Decoding KRAS mutation in non-small cell lung cancer patients receiving immunotherapy: A retrospective institutional comparison and literature review.Lung Cancer. 2025 Jan;199:108051. doi: 10.1016/j.lungcan.2024.108051. Epub 2024 Dec 9. Lung Cancer. 2025. PMID: 39740426 Review.
-
KRAS mutation predict response and outcome in advanced non-small cell lung carcinoma without driver alterations receiving PD-1 blockade immunotherapy combined with platinum-based chemotherapy: a retrospective cohort study from China.Transl Lung Cancer Res. 2022 Oct;11(10):2136-2147. doi: 10.21037/tlcr-22-655. Transl Lung Cancer Res. 2022. PMID: 36386464 Free PMC article.
-
Efficacy of Immune Checkpoint Inhibitors in KRAS-Mutant Non-Small Cell Lung Cancer (NSCLC).J Thorac Oncol. 2019 Jun;14(6):1095-1101. doi: 10.1016/j.jtho.2019.01.011. Epub 2019 Feb 6. J Thorac Oncol. 2019. PMID: 30738221
-
The landscape of immune checkpoint inhibitor plus chemotherapy versus immunotherapy for advanced non-small-cell lung cancer: A systematic review and meta-analysis.J Cell Physiol. 2020 May;235(5):4913-4927. doi: 10.1002/jcp.29371. Epub 2019 Nov 6. J Cell Physiol. 2020. PMID: 31693178 Free PMC article.
Cited by
-
Analysis of the immune microenvironment in colorectal cancer with different KRAS gene subtypes.BMC Cancer. 2025 Aug 4;25(1):1267. doi: 10.1186/s12885-025-14660-5. BMC Cancer. 2025. PMID: 40759935 Free PMC article.
-
KRAS Mutations as Predictive Biomarkers for First-Line Immune Checkpoint Inhibitor Monotherapy in Advanced NSCLC: A Systematic Review and Meta-Analysis.Curr Oncol. 2025 Jun 19;32(6):365. doi: 10.3390/curroncol32060365. Curr Oncol. 2025. PMID: 40558308 Free PMC article. Review.
-
Comparative analysis of PD-L1 expression and molecular alterations in primary versus metastatic lung adenocarcinoma: a real-world study in China.Front Oncol. 2024 Sep 11;14:1393686. doi: 10.3389/fonc.2024.1393686. eCollection 2024. Front Oncol. 2024. PMID: 39323996 Free PMC article.
-
KCTD10 inhibits lung cancer metastasis and angiogenesis via ubiquitin-mediated β-catenin degradation.Front Immunol. 2025 Aug 12;16:1630311. doi: 10.3389/fimmu.2025.1630311. eCollection 2025. Front Immunol. 2025. PMID: 40873559 Free PMC article.
-
Long-term survival with pemetrexed-based chemotherapy in a patient with metastatic lung adenocarcinoma of unclear primary origin harboring MTHFR C677T(T/T) mutation: a case report.Front Oncol. 2025 Jan 21;14:1435357. doi: 10.3389/fonc.2024.1435357. eCollection 2024. Front Oncol. 2025. PMID: 39906671 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
