Differential impact of intratumor heterogeneity (ITH) on survival outcomes in early-stage lung squamous and adenocarcinoma based on tumor mutational burden (TMB)
- PMID: 39118891
- PMCID: PMC11304137
- DOI: 10.21037/tlcr-24-226
Differential impact of intratumor heterogeneity (ITH) on survival outcomes in early-stage lung squamous and adenocarcinoma based on tumor mutational burden (TMB)
Abstract
Background: Molecular biomarkers are reshaping patient stratification and treatment decisions, yet their precise use and best implementation remain uncertain. Intratumor heterogeneity (ITH), an area of increasing research interest with prognostic value across various conditions, lacks defined clinical relevance in certain non-small cell lung cancer (NSCLC) subtypes. Exploring the relationship between ITH and tumor mutational burden (TMB) is crucial, as their interplay might reveal distinct patient subgroups. This study evaluates how the ITH-TMB dynamic affects prognosis across the two main histological subtypes of NSCLC, squamous cell and adenocarcinoma, with a specific focus on early-stage cases to address their highly unmet clinical needs.
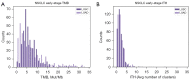
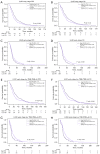
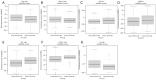
Methods: We stratify a cohort of 741 early-stage NSCLC patients from The Cancer Genome Atlas (TCGA) based on ITH and TMB and evaluate differences in clinical outcomes. Additionally, we compare driver mutations and the tumor microenvironment (TME) between high and low ITH groups.
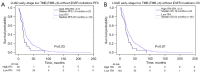
Results: In lung squamous cell carcinoma (LUSC), high ITH predicts an extended progression-free survival (PFS) (median: 21 vs. 14 months, P=0.01), while in lung adenocarcinoma (LUAD), high ITH predicts a reduced PFS (median: 15 vs. 20 months, P=0.04). This relationship is driven by the low TMB subset of patients. Additionally, we found that CD8 T cells were enriched in better-performing subgroups, regardless of histologic subtype or ITH status.
Conclusions: There are significant differences in clinical outcomes, driver mutations, and the TME between high and low ITH groups among early-stage NSCLC patients. These differences may have treatment implications, necessitating further validation in other NSCLC datasets.
Keywords: Non-small cell lung cancer (NSCLC); intratumor heterogeneity (ITH); molecular biomarkers; tumor mutational burden (TMB).
2024 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-226/coif). Y.K.C. reports that he received grants or contracts from AbbVie, BMS, Biodesix, Freenome, PictureHealth, and Predicine; consulting fees from Roche/Genentech, AstraZeneca, Foundation Medicine, Neogenomics, Guardant Health, Boehringer Ingelheim, Biodesix, ImmuneOncia, Lilly Oncology, Merck, Takeda, Lunit, Jazz Pharmaceutical, Tempus, BMS, Regeneron, NeoImmunTech, Esai; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Roche/Genentech, AstraZeneca, Foundation Medicine, Neogenomics, Guardant Health, Boehringer Ingelheim, Biodesix, ImmuneOncia, Lilly Oncology, Merck, Takeda, Lunit, Jazz Pharmaceutical, Tempus, BMS, Regeneron, NeoImmunTech, Esai. The other authors have no conflicts of interest to declare.
Figures
Similar articles
-
Intratumor heterogeneity comparison among different subtypes of non-small-cell lung cancer through multi-region tissue and matched ctDNA sequencing.Mol Cancer. 2019 Jan 9;18(1):7. doi: 10.1186/s12943-019-0939-9. Mol Cancer. 2019. PMID: 30626401 Free PMC article.
-
Comparison of the somatic genomic landscape between central- and peripheral-type non-small cell lung cancer.Lung Cancer. 2024 Jan;187:107439. doi: 10.1016/j.lungcan.2023.107439. Epub 2023 Dec 12. Lung Cancer. 2024. PMID: 38113653
-
An Intratumor Heterogeneity-Related Signature for Predicting Prognosis, Immune Landscape, and Chemotherapy Response in Colon Adenocarcinoma.Front Med (Lausanne). 2022 Jul 7;9:925661. doi: 10.3389/fmed.2022.925661. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35872794 Free PMC article.
-
Intratumor Heterogeneity and Antitumor Immunity Shape One Another Bidirectionally.Clin Cancer Res. 2022 Jul 15;28(14):2994-3001. doi: 10.1158/1078-0432.CCR-21-1355. Clin Cancer Res. 2022. PMID: 35380639 Free PMC article. Review.
-
Intratumor Heterogeneity as a Prognostic Factor in Solid Tumors: A Systematic Review and Meta-Analysis.Front Oncol. 2021 Oct 15;11:744064. doi: 10.3389/fonc.2021.744064. eCollection 2021. Front Oncol. 2021. PMID: 34722299 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
