Design, synthesis, and bioevaluation of diarylpyrimidine derivatives as novel microtubule destabilizers
- PMID: 39119517
- PMCID: PMC11306069
- DOI: 10.3389/fchem.2024.1447831
Design, synthesis, and bioevaluation of diarylpyrimidine derivatives as novel microtubule destabilizers
Abstract
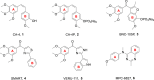
In this work, a series of new diarylpyrimidine derivatives as microtubule destabilizers were designed, synthesized, and evaluated for anticancer activities. Based on restriction configuration strategy, we introduced the pyrimidine moiety containing the hydrogen-bond acceptors as cis-olefin bond of CA-4 analogs to improve structural stability. Compounds 11a-t exerted antiproliferative activities against three human cancer cell lines (SGC-7901, HeLa, and MCF-7), due to tubulin polymerization inhibition, showing high selectivity toward cancer cells in comparison with non-tumoral HSF cells, as evidenced by MTT assays. In mechanistic investigations, compound 11s remarkably inhibited tubulin polymerization and disorganized microtubule in SGC-7901 cells by binding to tubulin. Moreover, 11s caused G2/M phase cell cycle arrest in SGC-7901 cells in a concentration-dependent manner. Furthermore, molecular modeling analysis revealed that 11s interacts with tubulin through binding to the colchicine site. In addition, the prediction of physicochemical properties disclosed that 11s conformed well to the Lipinski's rule of five. This work offered a fresh viewpoint for the discovery of new tubulin-targeting anticancer drugs.
Keywords: antiproliferative activity; combretastatin A-4; microtubule destabilizer; molecular docking; pyrimidine.
Copyright © 2024 Xiu, Zhang, Yang, Shi, Xing and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources

