Developing long bones respond to surrounding tissues by trans-pairing of periosteal osteoclasts and endocortical osteoblasts
- PMID: 39119717
- PMCID: PMC11423808
- DOI: 10.1242/dev.202194
Developing long bones respond to surrounding tissues by trans-pairing of periosteal osteoclasts and endocortical osteoblasts
Abstract
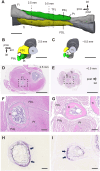
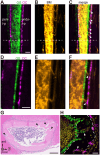
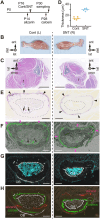
Developing long bones alter their shape while maintaining uniform cortical thickness via coordinated activity of bone-forming osteoblasts and bone-resorbing osteoclasts at periosteal and endosteal surfaces, a process we designate trans-pairing. Two types of trans-pairing shift cortical bone in opposite orientations: peri-forming trans-pairing (peri-t-p) increases bone marrow space and endo-forming trans-pairing (endo-t-p) decreases it, via paired activity of bone resorption and formation across the cortex. Here, we focused on endo-t-p in growing bones. Analysis of endo-t-p activity in the cortex of mouse fibulae revealed osteoclasts under the periosteum compressed by muscles, and expression of RANKL in periosteal cells of the cambium layer. Furthermore, mature osteoblasts were localized on the endosteum, while preosteoblasts were at the periosteum and within cortical canals. X-ray tomographic microscopy revealed the presence of cortical canals more closely associated with endo- than with peri-t-p. Sciatic nerve transection followed by muscle atrophy and unloading induced circumferential endo-t-p with concomitant spread of cortical canals. Such canals likely supply the endosteum with preosteoblasts from the periosteum under endo-t-p, allowing bone shape to change in response to mechanical stress or nerve injury.
Keywords: Cortical canals; Modeling drift; Osteoblasts; Osteoclasts; Sciatic nerve transection; Trans-cortical vessels.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





References
-
- Allen, M. R. and Burr, D. B. (2019). Chapter 5 - Bone growth, modeling, and remodeling. In Basic and Applied Bone Biology (ed. Allen M. R. and Burr D. B.) pp. 85-100. Academic Press.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

