Efficacy and prognostic factors of COVID-19 vaccine in patients with hepatocellular carcinoma: Analysis of data from a prospective cohort study
- PMID: 39119737
- PMCID: PMC11310663
- DOI: 10.1002/cam4.70068
Efficacy and prognostic factors of COVID-19 vaccine in patients with hepatocellular carcinoma: Analysis of data from a prospective cohort study
Abstract
Background: The efficacy of coronavirus disease 2019 (COVID-19) vaccines in preventing SARS-CoV-2 infection in patients with hepatocellular carcinoma (HCC) is not clear.
Methods: From January 2022 to October 2022, patients diagnosed with HCC in a prospective, multicenter, observational cohort were analyzed.
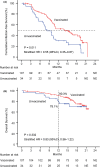
Results: One hundred and forty-one patients with (n = 107) or without COVID-19 vaccination (n = 34) were included. The number of patients with severe or very severe infection was relatively lower in the vaccinated group (3.7% vs. 11.8%, p = 0.096). Median infection-free survival in the vaccinated group (14.0 vs. 8.3 months, p = 0.010) was significantly longer than that in the unvaccinated group. COVID-19 vaccination (hazard ratio (HR) HR = 0.47), European Cooperative Oncology Group performance score = 0 (HR = 2.06), and extrahepatic spread (HR = 0.28) were found to be the independent predictive factors for infection-free survival.
Conclusion: COVID-19 vaccines could effectively reduce the SARS-Cov-2 infection in patients with HCC.
Keywords: clinical observations; hepatocellular carcinoma; prognosis; vaccine.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All of the authors have no conflict of interests.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
-
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82330061/National Natural Science Fund of China
- 2021-I2M-1-015-3/Chinese Academy of Medical Sciences Initiative for Innovative Medicine
- GWJJ2024100302/National Health Commission Capacity Building And Continuing Education Center Annual Project
- LC2021C03/Beijing Hope Run Special Fund of Cancer Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous