Hypocretin-1/Hypocretin Receptor 1 Regulates Neuroplasticity and Cognitive Function through Hippocampal Lactate Homeostasis in Depressed Model
- PMID: 39119889
- PMCID: PMC11481194
- DOI: 10.1002/advs.202405354
Hypocretin-1/Hypocretin Receptor 1 Regulates Neuroplasticity and Cognitive Function through Hippocampal Lactate Homeostasis in Depressed Model
Abstract
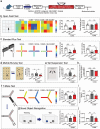
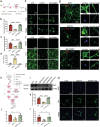
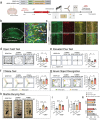
Cognitive dysfunction is not only a common symptom of major depressive disorder, but also a more common residual symptom after antidepressant treatment and a risk factor for chronic and recurrent disease. The disruption of hypocretin regulation is known to be associated with depression, however, their exact correlation is remains to be elucidated. Hypocretin-1 levels are increased in the plasma and hypothalamus from chronic unpredictable mild stress (CUMS) model mice. Excessive hypocretin-1 conducted with hypocretin receptor 1 (HCRTR1) reduced lactate production and brain-derived neurotrophic factor (BDNF) expression by hypoxia-inducible factor-1α (HIF-1α), thus impairing adult hippocampal neuroplasticity, and cognitive impairment in CUMS model. Subsequently, it is found that HCRTR1 antagonists can reverse these changes. The direct effect of hypocretin-1 on hippocampal lactate production and cognitive behavior is further confirmed by intraventricular injection of hypocretin-1 and microPET-CT in rats. In addition, these mechanisms are further validated in astrocytes and neurons in vitro. Moreover, these phenotypes and changes in molecules of lactate transport pathway can be duplicated by specifically knockdown of HCRTR1 in hippocampal astrocytes. In summary, the results provide molecular and functional insights for involvement of hypocretin-1-HCRTR1 in altered cognitive function in depression.
Keywords: cognition; depression; hypocretin‐1; hypoxia‐inducible factor‐1α; lactate.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
