Central Role of Hypothalamic Circuits for Acupuncture's Anti-Parkinsonian Effects
- PMID: 39119926
- PMCID: PMC11578310
- DOI: 10.1002/advs.202403245
Central Role of Hypothalamic Circuits for Acupuncture's Anti-Parkinsonian Effects
Abstract
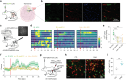
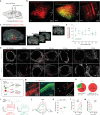
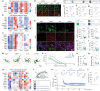
Despite clinical data stretching over millennia, the neurobiological basis of the effectiveness of acupuncture in treating diseases of the central nervous system has remained elusive. Here, using an established model of acupuncture treatment in Parkinson's disease (PD) model mice, we show that peripheral acupuncture stimulation activates hypothalamic melanin-concentrating hormone (MCH) neurons via nerve conduction. We further identify two separate neural pathways originating from anatomically and electrophysiologically distinct MCH neuronal subpopulations, projecting to the substantia nigra and hippocampus, respectively. Through chemogenetic manipulation specifically targeting these MCH projections, their respective roles in mediating the acupuncture-induced motor recovery and memory improvements following PD onset are demonstrated, as well as the underlying mechanisms mediating recovery from dopaminergic neurodegeneration, reactive gliosis, and impaired hippocampal synaptic plasticity. Collectively, these MCH neurons constitute not only a circuit-based explanation for the therapeutic effectiveness of traditional acupuncture, but also a potential cellular target for treating both motor and non-motor PD symptoms.
Keywords: Parkinson's disease (PD); acupuncture; hypothalamus; melanin‐concentrating hormone (MCH); motor and non‐motor symptoms; neural circuitry.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Tysnes O. B., Storstein A., J. Neural Transm. 2017, 124, 901. - PubMed
-
- Armstrong M. J., Okun M. S., JAMA, J. Am. Med. Assoc. 2020, 323, 548. - PubMed
-
- a) Park H. K., Lee H., Neurol Res. 2010, 32, 3;
- b) Kaptchuk T. J., Ann. Intern. Med. 2002, 136, 374. - PubMed
-
- a) Li K., Xu S., Wang R., Zou X., Liu H., Fan C., Li J., Li G., Wu Y., Ma X., Chen Y., Hu C., Liu X., Yuan C., Ye Q., Dai M., Wu L., Wang Z., Wu H., EClinicalMedicine 2023, 56, 101814; - PMC - PubMed
- b) Pereira C. R., Machado J., Rodrigues J., de Oliveira N. M., Criado M. B., Greten H. J., Healthcare (Basel) 2022, 10; - PMC - PubMed
- c) Noh H., Kwon S., Cho S. Y., Jung W. S., Moon S. K., Park J. M., Ko C. N., Park S. U., Complement Ther. Med. 2017, 34, 86. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
