Using exploratory pharmacokinetic and pharmacodynamic analyses to predict the probability of flu-like symptoms in healthy volunteers and patients with chronic hepatitis B treated with the toll-like receptor 7 agonist ruzotolimod
- PMID: 39119977
- PMCID: PMC11310849
- DOI: 10.1111/cts.13896
Using exploratory pharmacokinetic and pharmacodynamic analyses to predict the probability of flu-like symptoms in healthy volunteers and patients with chronic hepatitis B treated with the toll-like receptor 7 agonist ruzotolimod
Abstract
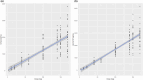
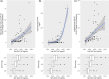
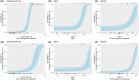
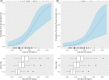
Ruzotolimod (Toll-like receptor 7 (TLR7) agonist, RG7854) is an oral, small molecule immuno-modulator activating the TLR 7 and is being evaluated in patients with CHB. As with other TLR7 agonists, the study drug-related adverse events of flu-like symptoms have been reported in some participants during phase I studies with ruzotolimod. An exploratory analysis of the relationship between pharmacokinetic (PK)/pharmacodynamic (PD) and flu-like symptoms was performed in participants from two phase I studies including both healthy volunteers and NUC-suppressed CHB patients who received either single or multiple ascending doses of orally administered ruzotolimod. Linear and logistic regression were used to explore potential relationships between dose, flu-like symptoms, PK, and PD. Generalized linear regression was performed to predict the probability of flu-like symptoms of all intensities at different RO7011785 (the active metabolite of the double prodrug ruzotolimod) PK exposure. This analysis showed that single or multiple doses of ruzotolimod at ⩾100 mg, the immune PD (IFN-α, neopterin, IP-10, and the transcriptional expression of ISG15, OAS-1, MX1, and TLR7) responses increase with the RO7011785 PK exposure, which increases linearly with the doses from 3 mg to 170 mg of ruzotolimod. The analysis also showed that the probability of flu-like symptoms occurrence increases with PD responses (IFN-α and IP-10). Dose reduction of ruzotolimod can be an effective way to reduce the magnitude of PD response, thus reducing the probability of study drug-related flu-like symptoms occurrence at all intensity in the participants who are highly sensitive to PD activation and intolerant to flu-like symptoms.
© 2024 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Q.J., Y.C., D.D., S.R., R.U., K.G. and M.T. are employees of F. Hoffmann‐La Roche, and Y.Z., J.F.G. and Y.J. are former employees of F. Hoffmann‐La Roche. All of them have no competing interests for this work.
Figures

References
-
- World Health Organization . Hepatitis B . Updated on 18 July. https://www.who.int/news‐room/fact‐sheets/detail/hepatitis‐b
-
- Mehta P, Reddivari AKR. Hepatitis. StatPearls; 2020.
-
- Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. J Hepatol. 2017;67:847‐861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

