Sex Differences Affect the NRF2 Signaling Pathway in the Early Phase of Liver Steatosis: A High-Fat-Diet-Fed Rat Model Supplemented with Liquid Fructose
- PMID: 39120278
- PMCID: PMC11312139
- DOI: 10.3390/cells13151247
Sex Differences Affect the NRF2 Signaling Pathway in the Early Phase of Liver Steatosis: A High-Fat-Diet-Fed Rat Model Supplemented with Liquid Fructose
Abstract
Sex differences may play a role in the etiopathogenesis and severity of metabolic dysfunction-associated steatotic liver disease (MASLD), a disorder characterized by excessive fat accumulation associated with increased inflammation and oxidative stress. We previously observed the development of steatosis specifically in female rats fed a high-fat diet enriched with liquid fructose (HFHFr) for 12 weeks. The aim of this study was to better characterize the observed sex differences by focusing on the antioxidant and cytoprotective pathways related to the KEAP1/NRF2 axis. The KEAP1/NRF2 signaling pathway, autophagy process (LC3B and LAMP2), and endoplasmic reticulum stress response (XBP1) were analyzed in liver homogenates in male and female rats that were fed a 12-week HFHFr diet. In females, the HFHFr diet resulted in the initial activation of the KEAP1/NRF2 pathway, which was not followed by the modulation of downstream molecular targets; this was possibly due to the increase in KEAP1 levels preventing the nuclear translocation of NRF2 despite its cytosolic increase. Interestingly, while in both sexes the HFHFr diet resulted in an increase in the levels of LC3BII/LC3BI, a marker of autophagosome formation, only males showed a significant upregulation of LAMP2 and XBP1s; this did not occur in females, suggesting impaired autophagic flux in this sex. Overall, our results suggest that males are characterized by a greater ability to cope with an HFHFr metabolic stimulus mainly through an autophagic-mediated proteostatic process while in females, this is impaired. This might depend at least in part upon the fine modulation of the cytoprotective and antioxidant KEAP1/NRF2 pathway resulting in sex differences in the occurrence and severity of MASLD. These results should be considered to design effective therapeutics for MASLD.
Keywords: KEAP1; NRF2; antioxidants; autophagy; endoplasmic reticulum stress response; fructose; high-fat diet; liver steatosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

 ) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NRF2 quantification at the gene expression level (a), at protein levels as the total NRF2 (b), in the cytosolic (c) and nuclear fractions, and (d) in the liver of female and male animals after being fed the HFHFr diet and control diet. (c,d) Western blot representative images and densitometric analysis. (e,f) KEAP1 quantification at the gene expression level and protein level. Comparison among groups was carried out via 2-way ANOVA using the HFHFr diet and sex configuration. A significant main effect of diet is indicated by §; a significant main effect of sex is indicated by #; A Tukey post hoc test or independent t-test was conducted for group comparisons and are indicated by *. Significant differences are indicated by p-values: *,§ p < 0.032; ## p < 0.0021; *** p < 0.0002; **** p < 0.0001.
) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NRF2 quantification at the gene expression level (a), at protein levels as the total NRF2 (b), in the cytosolic (c) and nuclear fractions, and (d) in the liver of female and male animals after being fed the HFHFr diet and control diet. (c,d) Western blot representative images and densitometric analysis. (e,f) KEAP1 quantification at the gene expression level and protein level. Comparison among groups was carried out via 2-way ANOVA using the HFHFr diet and sex configuration. A significant main effect of diet is indicated by §; a significant main effect of sex is indicated by #; A Tukey post hoc test or independent t-test was conducted for group comparisons and are indicated by *. Significant differences are indicated by p-values: *,§ p < 0.032; ## p < 0.0021; *** p < 0.0002; **** p < 0.0001.
 ) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NQO1 and HO1 quantification at the gene expression level (a,b), and at protein levels (c), in the liver of female and male animals after being fed the HFHFr diet and control diet. (c) Western blot representative images and densitometric analysis. A significant main effect of sex is indicated by # and the main effect of diet is indicated by §. Significant differences are indicated by p-values: §,# p < 0.032.
) HFHFr (high-fat diet devoid of cholesterol, plus 10% fructose as beverage); each pool was obtained from mixing equal amounts of two individual tissue samples. The box plot graphs show medians and inter-quartile ranges of NQO1 and HO1 quantification at the gene expression level (a,b), and at protein levels (c), in the liver of female and male animals after being fed the HFHFr diet and control diet. (c) Western blot representative images and densitometric analysis. A significant main effect of sex is indicated by # and the main effect of diet is indicated by §. Significant differences are indicated by p-values: §,# p < 0.032.
 ) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. Western blot representative images and densitometric analysis of (a) p62 and LC3B and (b) LAMP2 are shown. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). A significant main effect of diet is indicated by §. Tukey post hoc tests were conducted for group comparisons, and the results are indicated by *. Significant differences are indicated by p-values: §§,** p < 0.0021; *** p < 0.0002.
) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. Western blot representative images and densitometric analysis of (a) p62 and LC3B and (b) LAMP2 are shown. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). A significant main effect of diet is indicated by §. Tukey post hoc tests were conducted for group comparisons, and the results are indicated by *. Significant differences are indicated by p-values: §§,** p < 0.0021; *** p < 0.0002.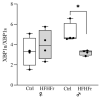
 ) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). Significant differences are indicated by p-values: * p < 0.05.
) HFHFr (high-fat, devoid of cholesterol, plus 10% fructose as beverage) diet. We used eight animals for each experimental group and prepared four different pooled samples for each experimental condition; each pool was obtained by mixing equal amounts of two individual tissue samples (final N = 4). Significant differences are indicated by p-values: * p < 0.05.
References
-
- Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wai-Sun Wong V., Dufour J.F., Schattenberg J.M., et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. - DOI - PubMed
-
- Kleiner D.E., Brunt E.M., Wilson L.A., Behling C., Guy C., Contos M., Cummings O., Yeh M., Gill R., Chalasani N., et al. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw. Open. 2019;2:e1912565. doi: 10.1001/jamanetworkopen.2019.12565. - DOI - PMC - PubMed
-
- Brunt E.M., Kleiner D.E., Wilson L.A., Sanyal A.J., Neuschwander-Tetri B.A. Improvements in Histologic Features and Diagnosis Associated with Improvement in Fibrosis in Nonalcoholic Steatohepatitis: Results From the Nonalcoholic Steatohepatitis Clinical Research Network Treatment Trials. Hepatology. 2019;70:522–531. doi: 10.1002/hep.30418. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

