Enzyme Is the Name-Adapter Is the Game
- PMID: 39120280
- PMCID: PMC11311582
- DOI: 10.3390/cells13151249
Enzyme Is the Name-Adapter Is the Game
Abstract
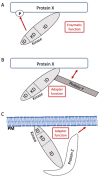
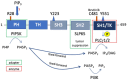
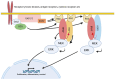
Signaling proteins in eukaryotes usually comprise a catalytic domain coupled to one or several interaction domains, such as SH2 and SH3 domains. An additional class of proteins critically involved in cellular communication are adapter or scaffold proteins, which fulfill their purely non-enzymatic functions by organizing protein-protein interactions. Intriguingly, certain signaling enzymes, e.g., kinases and phosphatases, have been demonstrated to promote particular cellular functions by means of their interaction domains only. In this review, we will refer to such a function as "the adapter function of an enzyme". Though many stories can be told, we will concentrate on several proteins executing critical adapter functions in cells of the immune system, such as Bruton´s tyrosine kinase (BTK), phosphatidylinositol 3-kinase (PI3K), and SH2-containing inositol phosphatase 1 (SHIP1), as well as in cancer cells, such as proteins of the rat sarcoma/extracellular signal-regulated kinase (RAS/ERK) mitogen-activated protein kinase (MAPK) pathway. We will also discuss how these adaptor functions of enzymes determine or even undermine the efficacy of targeted therapy compounds, such as ATP-competitive kinase inhibitors. Thereby, we are highlighting the need to develop pharmacological approaches, such as proteolysis-targeting chimeras (PROTACs), that eliminate the entire protein, and thus both enzymatic and adapter functions of the signaling protein. We also review how genetic knock-out and knock-in approaches can be leveraged to identify adaptor functions of signaling proteins.
Keywords: ErbB3/HER3; KSR1; SHP2; cancer; dimerization; immunodeficiency; inflammatory diseases; protein complexes; pseudokinase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

