Regulatory B Cells Expressing Granzyme B from Tolerant Renal Transplant Patients: Highly Differentiated B Cells with a Unique Pathway with a Specific Regulatory Profile and Strong Interactions with Immune System Cells
- PMID: 39120317
- PMCID: PMC11311295
- DOI: 10.3390/cells13151287
Regulatory B Cells Expressing Granzyme B from Tolerant Renal Transplant Patients: Highly Differentiated B Cells with a Unique Pathway with a Specific Regulatory Profile and Strong Interactions with Immune System Cells
Abstract
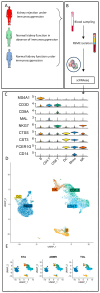
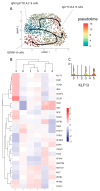
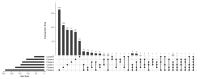
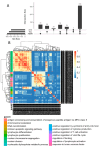
The aim of our study was to determine whether granzyme B-expressing regulatory B cells (GZMB+ B cells) are enriched in the blood of transplant patients with renal graft tolerance. To achieve this goal, we analysed two single-cell RNA sequencing (scRNAseq) datasets: (1) peripheral blood mononuclear cells (PBMCs), including GZMB+ B cells from renal transplant patients, i.e., patients with stable graft function on conventional immunosuppressive treatment (STA, n = 3), drug-free tolerant patients (TOL, n = 3), and patients with antibody-mediated rejection (ABMR, n = 3), and (2) ex-vivo-induced GZMB+ B cells from these groups. In the patient PBMCs, we first showed that natural GZMB+ B cells were enriched in genes specific to Natural Killer (NK) cells (such as NKG7 and KLRD1) and regulatory B cells (such as GZMB, IL10, and CCL4). We performed a pseudotemporal trajectory analysis of natural GZMB+ B cells and showed that they were highly differentiated B cells with a trajectory that is very different from that of conventional memory B cells and linked to the transcription factor KLF13. By specifically analysing GZMB+ natural B cells in TOLs, we found that these cells had a very specific transcriptomic profile associated with a reduction in the expression of HLA molecules, apoptosis, and the inflammatory response (in general) in the blood and that this signature was conserved after ex vivo induction, with the induction of genes associated with migration processes, such as CCR7, CCL3, or CCL4. An analysis of receptor/ligand interactions between these GZMB+/- natural B cells and all of the immune cells present in PBMCs also demonstrated that GZMB+ B cells were the B cells that carried the most ligands and had the most interactions with other immune cells, particularly in tolerant patients. Finally, we showed that these GZMB+ B cells were able to infiltrate the graft under inflammatory conditions, thus suggesting that they can act in locations where immune events occur.
Keywords: B cells; kidney; lymphocyte; regulation; tolerance; transplantation.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures





References
-
- Benítez C., Londoño M.-C., Miquel R., Manzia T.-M., Abraldes J.G., Lozano J.-J., Martínez-Llordella M., López M., Angelico R., Bohne F., et al. Prospective Multicenter Clinical Trial of Immunosuppressive Drug Withdrawal in Stable Adult Liver Transplant Recipients: Hepatology. Hepatology. 2013;58:1824–1835. doi: 10.1002/hep.26426. - DOI - PubMed
-
- Taubert R., Danger R., Londoño M.-C., Christakoudi S., Martinez-Picola M., Rimola A., Manns M.P., Sánchez-Fueyo A., Jaeckel E. Hepatic Infiltrates in Operational Tolerant Patients After Liver Transplantation Show Enrichment of Regulatory T Cells Before Proinflammatory Genes Are Downregulated. Am. J. Transplant. 2016;16:1285–1293. doi: 10.1111/ajt.13617. - DOI - PubMed
-
- Guinn M.T., Szuter E.S., Yokose T., Ge J., Rosales I.A., Chetal K., Sadreyev R.I., Cuenca A.G., Kreisel D., Sage P.T., et al. Intragraft B Cell Differentiation during the Development of Tolerance to Kidney Allografts Is Associated with a Regulatory B Cell Signature Revealed by Single Cell Transcriptomics. Am. J. Transplant. 2023;23:1319–1330. doi: 10.1016/j.ajt.2023.05.036. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

