Carbon Nanotube Immunotoxicity in Alveolar Epithelial Type II Cells Is Mediated by Physical Contact-Independent Cell-Cell Interaction with Macrophages as Demonstrated in an Optimized Air-Liquid Interface (ALI) Coculture Model
- PMID: 39120378
- PMCID: PMC11314342
- DOI: 10.3390/nano14151273
Carbon Nanotube Immunotoxicity in Alveolar Epithelial Type II Cells Is Mediated by Physical Contact-Independent Cell-Cell Interaction with Macrophages as Demonstrated in an Optimized Air-Liquid Interface (ALI) Coculture Model
Abstract
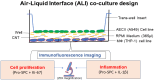
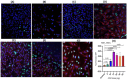
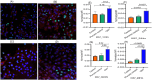
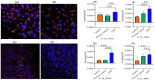
There is a need for the assessment of respiratory hazard potential and mode of action of carbon nanotubes (CNTs) before their approval for technological or medical applications. In CNT-exposed lungs, both alveolar macrophages (MФs), which phagocytose CNTs, and alveolar epithelial type II cells (AECII cells), which show tissue injury, are impacted but cell-cell interactions between them and the impacted mechanisms are unclear. To investigate this, we first optimized an air-liquid interface (ALI) transwell coculture of human AECII cell line A549 (upper chamber) and human monocyte cell line THP-1 derived macrophages (lower chamber) in a 12-well culture by exposing macrophages to CNTs at varying doses (5-60 ng/well) for 12-48 h and measuring the epithelial response markers for cell differentiation/maturation (proSP-C), proliferation (Ki-67), and inflammation (IL-1β). In optimal ALI epithelial-macrophage coculture (3:1 ratio), expression of Ki-67 in AECII cells showed dose dependence, peaking at 15 ng/well CNT dose; the Ki-67 and IL-1β responses were detectable within 12 h, peaking at 24-36 h in a time-course. Using the optimized ALI transwell coculture set up with and without macrophages, we demonstrated that direct interaction between CNTs and MФs, but not a physical cell-cell contact between MФ and AECII cells, was essential for inducing immunotoxicity (proliferative and inflammatory responses) in the AECII cells.
Keywords: MWCNT; air–liquid interface; alveolar epithelial cells; alveolar macrophages; carbon nanotube; coculture; immunotoxicity; inhalation toxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

