Enhanced Fenton Degradation of Tetracycline over Cerium-Doped MIL88-A/g-C3N4: Catalytic Performance and Mechanism
- PMID: 39120389
- PMCID: PMC11313986
- DOI: 10.3390/nano14151282
Enhanced Fenton Degradation of Tetracycline over Cerium-Doped MIL88-A/g-C3N4: Catalytic Performance and Mechanism
Abstract
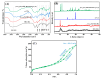
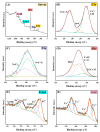
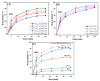
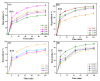
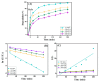
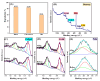
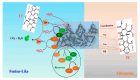
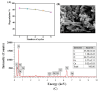
Since enormous amounts of antibiotics are consumed daily by millions of patients all over the world, tons of pharmaceutical residuals reach aquatic bodies. Accordingly, our study adopted the Fenton catalytic degradation approach to conquer such detrimental pollutants. (Ce0.33Fe) MIL-88A was fabricated by the hydrothermal method; then, it was supported on the surface of g-C3N4 sheets using the post-synthetic approach to yield a heterogeneous Fenton-like (Ce0.33Fe) MIL-88A/10%g-C3N4 catalyst for degrading the tetracycline hydrochloride drug. The physicochemical characteristics of the catalyst were analyzed using FT-IR, SEM-EDX, XRD, BET, SEM, and XPS. The pH level, the H2O2 concentration, the reaction temperature, the catalyst dose, and the initial TC concentration were all examined as influencing factors of TC degradation efficiency. Approximately 92.44% of the TC was degraded within 100 min under optimal conditions: pH = 7, catalyst dosage = 0.01 g, H2O2 concentration = 100 mg/L, temperature = 25 °C, and TC concentration = 50 mg/L. It is noteworthy that the practical outcomes revealed how the Fenton-like process and adsorption work together. The degradation data were well-inspected by first-order and second-order models to define the reaction rate. The synergistic interaction between the (Ce0.33Fe) MIL-88A/10%g-C3N4 components produces a continuous redox cycle of two active metal species and the electron-rich source of g-C3N4. The quenching test demonstrates that •OH is the primary active species for degrading TC in the H2O2-(Ce0.33Fe) MIL-88A/10%g-C3N4 system. The GC-MS spectrum elucidates the yielded intermediates from degrading the TC molecules.
Keywords: Ce-doping; Fenton-like degradation; GC-MS; XPS; degradation mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- El-Monaem E.M.A., Hosny M., Eltaweil A.S. Synergistic effect between adsorption and Fenton-like degradation on CoNiFe-LDH/ZIF-8 composite for efficient degradation of doxycycline. Chem. Eng. Sci. 2024;287:119707. doi: 10.1016/j.ces.2024.119707. - DOI
-
- Omer A.M., El-Monaem E.M.A., El-Subruiti G.M., El-Latif M.M.A., Eltaweil A.S. Fabrication of easy separable and reusable MIL-125(Ti)/MIL-53(Fe) binary MOF/CNT/Alginate composite microbeads for tetracycline removal from water bodies. Sci. Rep. 2021;11:23818. doi: 10.1038/s41598-021-03428-z. - DOI - PMC - PubMed
-
- Abdelfatah A.M., Fawzy M., El-Khouly M.E., Eltaweil A.S. Efficient adsorptive removal of tetracycline from aqueous solution using phytosynthesized nano-zero valent iron. J. Saudi Chem. Soc. 2021;25:101365. doi: 10.1016/j.jscs.2021.101365. - DOI
-
- El-Monaem E.M.A., Eltaweil A.S., Elshishini H.M., Hosny M., Alsoaud M.M.A., Attia N.F., El-Subruiti G.M., Omer A.M. Sustainable adsorptive removal of antibiotic residues by chitosan composites: An insight into current developments and future recommendations. Arab. J. Chem. 2022;15:103743. doi: 10.1016/j.arabjc.2022.103743. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

