Polyethylene Terephthalate Microplastics Generated from Disposable Water Bottles Induce Interferon Signaling Pathways in Mouse Lung Epithelial Cells
- PMID: 39120391
- PMCID: PMC11314056
- DOI: 10.3390/nano14151287
Polyethylene Terephthalate Microplastics Generated from Disposable Water Bottles Induce Interferon Signaling Pathways in Mouse Lung Epithelial Cells
Abstract
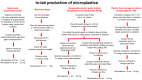
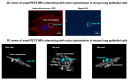
Microplastics (MPs) are present in ambient air in a respirable size fraction; however, their potential impact on human health via inhalation routes is not well documented. In the present study, methods for a lab-scale generation of MPs from regularly used and littered plastic articles were optimized. The toxicity of 11 different types of MPs, both commercially purchased and in-lab prepared MPs, was investigated in lung epithelial cells using cell viability, immune and inflammatory response, and genotoxicity endpoints. The underlying mechanisms were identified by microarray analysis. Although laborious, the laboratory-scale methods generated a sufficient quantity of well characterized MPs for toxicity testing. Of the 11 MPs tested, the small sized polyethylene terephthalate (PETE) MPs prepared from disposable water bottles induced the maximum toxicity. Specifically, the smaller size PETE MPs induced a robust activation of the interferon signaling pathway, implying that PETE MPs are perceived by cells by similar mechanisms as those employed to recognize pathogens. The PETE MPs of heterogenous size and shapes induced cell injury, triggering cell death, inflammatory cascade, and DNA damage, hallmark in vitro events indicative of potential in vivo tissue injury. The study establishes toxicity of specific types of plastic materials in micron and nano size.
Keywords: cytotoxicity; environmental exposure; genotoxicity; inhalation toxicology; micronuclei; microplastics; polyethylene terephthalate; transcriptomic.
Conflict of interest statement
The authors declare no conflicts of interest. The design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript; or the decision to publish the results were not influenced by the funder.
Figures









References
-
- Gregory M.R. Plastic ‘scrubbers’ in hand cleansers: A further (and minor) source for marine pollution identified. Mar. Pollut. Bull. 1996;32:867–871. doi: 10.1016/S0025-326X(96)00047-1. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

