HER2 Antibody-Drug Conjugates Are Active against Desmoplastic Small Round Cell Tumor
- PMID: 39120576
- PMCID: PMC11479846
- DOI: 10.1158/1078-0432.CCR-24-1835
HER2 Antibody-Drug Conjugates Are Active against Desmoplastic Small Round Cell Tumor
Abstract
Purpose: Desmoplastic small round cell tumor (DSRCT) is a rare but highly aggressive soft tissue sarcoma that arises in the abdominopelvic cavity of young males. Since the discovery of EWSR1::WT1 fusion as the driver of DSRCT, no actionable genomic alterations have been identified, limiting disease management to a combination of surgery, chemotherapy, and radiation, with very poor outcomes. Herein, we evaluated ERBB2/HER2 expression in DSRCT as a therapeutic target.
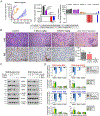
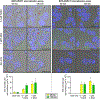
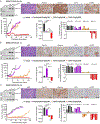
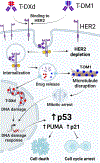
Experimental design: ERBB2/HER2 expression was assessed in clinical samples and patient-derived xenografts (PDX) using RNA sequencing, RT-qPCR, and a newly developed HER2 IHC assay (clone 29D8). Responses to HER2 antibody-drug conjugates (ADC)-trastuzumab deruxtecan (T-DXd) and trastuzumab emtansine-were evaluated in DSRCT PDX, cell line, and organoid models. Drug internalization was demonstrated by live microscopy. Apoptosis was evaluated by Western blotting and caspase activity assays.
Results: ERBB2/HER2 was detectable in DSRCT samples from patients and PDXs, with higher sensitivity RNA assays and improved IHC detectability using clone 29D8. Treatment of ERBB2/HER2-expressing DSRCT PDX, cell line, and organoid models with T-DXd or trastuzumab emtansine resulted in tumor regression. This therapeutic response was long-lasting in T-DXd-treated xenografts and was mediated by rapid HER2 ADC complex internalization and cytotoxicity, triggering p53-mediated apoptosis and growth arrest. Xenograft regression was associated with bystander payload effects triggering global tumor niche responses proportional to HER2 status.
Conclusions: ERBB2/HER2 is a therapeutic target in DSRCT. HER2 ADCs may represent novel options for managing this exceptionally aggressive sarcoma, possibly fulfilling an urgent and historically unmet need for more effective clinical therapy.
©2024 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest Statements
Tom Zhang, Christopher A. Febres-Aldana, Zebing Liu, Jenna-Marie Dix, Ryan Cheng, Raymond G. Dematteo, Allan JW Lui, Inna Khodos, Leo Gili, Marissa S. Mattar, Jeanine Lisanti, Charlene Kwong, Irina Linkov, Murray J. Tipping, Elisa de Stanchina, and Igor Odintsov report no potential conflicts of interest.
Romel Somwar received research grants from Helsinn Healthcare, SA, LOXO Oncology, Elevation Oncology, and Merus, all unrelated to this study.
Marc Ladanyi received advisory board compensation from Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Takeda, and Bayer, and research support from LOXO Oncology, Elevation Oncology, and Helsinn Healthcare.
Figures


References
-
- Gani F, Goel U, Canner JK, Meyer CF, Johnston FM. A national analysis of patterns of care and outcomes for adults diagnosed with desmoplastic small round cell tumors in the United States. J Surg Oncol. 2019;119:880–6. - PubMed
-
- Ladanyi M, Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res. 1994;54:2837–40. - PubMed
-
- Gerald WL, Haber DA. The EWS–WT1 gene fusion in desmoplastic small round cell tumor. Semin Cancer Biol. 2005;15:197–205. - PubMed
-
- Schmidt D, Köster E, Harms D. Intraabdominal desmoplastic small-cell tumor with divergent differentiation: Clinicopathological findings and DNA ploidy. Méd Pediatr Oncol. 1994;22:97–102. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

