Cracking the Code: Reprogramming the Genetic Script in Prokaryotes and Eukaryotes to Harness the Power of Noncanonical Amino Acids
- PMID: 39120726
- PMCID: PMC11441406
- DOI: 10.1021/acs.chemrev.3c00878
Cracking the Code: Reprogramming the Genetic Script in Prokaryotes and Eukaryotes to Harness the Power of Noncanonical Amino Acids
Abstract
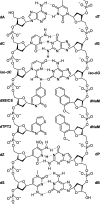
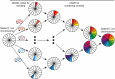
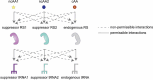
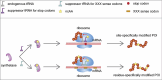
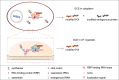
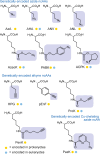
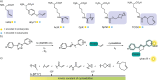
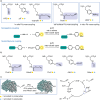
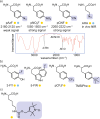
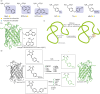
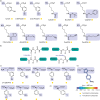
Over 500 natural and synthetic amino acids have been genetically encoded in the last two decades. Incorporating these noncanonical amino acids into proteins enables many powerful applications, ranging from basic research to biotechnology, materials science, and medicine. However, major challenges remain to unleash the full potential of genetic code expansion across disciplines. Here, we provide an overview of diverse genetic code expansion methodologies and systems and their final applications in prokaryotes and eukaryotes, represented by Escherichia coli and mammalian cells as the main workhorse model systems. We highlight the power of how new technologies can be first established in simple and then transferred to more complex systems. For example, whole-genome engineering provides an excellent platform in bacteria for enabling transcript-specific genetic code expansion without off-targets in the transcriptome. In contrast, the complexity of a eukaryotic cell poses challenges that require entirely new approaches, such as striving toward establishing novel base pairs or generating orthogonally translating organelles within living cells. We connect the milestones in expanding the genetic code of living cells for encoding novel chemical functionalities to the most recent scientific discoveries, from optimizing the physicochemical properties of noncanonical amino acids to the technological advancements for their in vivo incorporation. This journey offers a glimpse into the promising developments in the years to come.
Conflict of interest statement
The authors declare the following competing financial interest(s): E.A.L. holds several patents related to genetic code expansion and is a cofounder and consultant of Veraxa Biotech GmbH, a company specialized on generation of antibody drug conjugates via GCE.
Figures


















References
-
- Nirenberg M. W.; Jones O. W.; Leder P.; Clark B. F. C.; Sly W. S.; Pestka S. On the Coding of Genetic Information. Cold Spring Harb. Symp. Quant. Biol. 1963, 28, 549–557. 10.1101/SQB.1963.028.01.074. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

