Implementation of PET/CT in radiation oncology-a patterns-of-care analysis of the German Society of Nuclear Medicine and the German Society of Radiation Oncology
- PMID: 39120747
- PMCID: PMC11527913
- DOI: 10.1007/s00066-024-02260-4
Implementation of PET/CT in radiation oncology-a patterns-of-care analysis of the German Society of Nuclear Medicine and the German Society of Radiation Oncology
Abstract
Background: The use of positron-emission tomography (PET)/computed tomography (CT) in radiation therapy (RT) has increased. Radiation oncologists (RadOncs) have access to PET/CT with a variety of tracers for different tumor entities and use it for target volume definition. The German Society of Nuclear Medicine (DGN) and the German Society of Radiation Oncology (DEGRO) aimed to identify current patterns of care in order to improve interdisciplinary collaboration.
Methods: We created an online survey on participating RadOncs' use of PET tracers for different tumor entities and how they affect RT indication, dose prescription, and target volume definition. Further topics were reimbursement of PET/CT and organizational information (fixed timeslots and use of PET with an immobilization device [planning/RT-PET]). The survey contained 31 questions in German language (yes/no questions, multiple choice [MC] questions, multiple select [MS] questions, and free-text entry options). The survey was distributed twice via the DEGRO member mailing list.
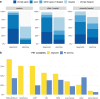
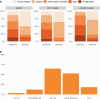
Results: During the survey period (May 22-August 7, 2023) a total of 156 RadOncs (13% of respondents) answered the survey. Among these, 59% reported access to diagnostic PET/CT within their organization/clinic and 24% have fixed timeslots for their patients. 37% of survey participants can perform RT-PET and 29% have the option of providing a dedicated RT technician for planning PET. Besides [18F]-fluorodeoxyglucose (FDG; mainly used in lung cancer: 95%), diagnostic prostate-specific membrane antigen (PSMA)-PET/CT for RT of prostate cancer is routinely used by 44% of participants (by 64% in salvage RT). Use of amino acid PET in brain tumors and somatostatin receptor PET in meningioma is low (19 and 25%, respectively). Scans are reimbursed through private (75%) or compulsory (55%) health insurance or as part of indications approved by the German Joint Federal Committee (Gemeinsamer Bundesausschuss; 59%). 98% of RadOncs agree that PET impacts target volume definition and 62% think that it impacts RT dose prescription.
Discussion: This is the first nationwide survey on the role of PET/CT for RT planning among RadOncs in Germany. We find high acceptance of PET results for treatment decisions and target volume definition. Planning PET comes with logistic challenges for different healthcare settings (e.g., private practices vs. university hospitals). The decision to request PET/CT is often based on the possibility of reimbursement.
Conclusion: PET/CT has become an important tool for RadOncs, with several indications. However, access is still limited at several sites, especially for dedicated RT-PET. This study aims to improve interdisciplinary cooperation and adequate implementation of current guidelines for the treatment of various tumor entities.
Keywords: Germany; PET/CT; Planning PET; Reimbursement; Survey.
© 2024. The Author(s).
Conflict of interest statement
L.M. Unterrainer discloses personal fees (speaker honorary and/or advisory boards fees) from Astellas Pharma Inc., Novartis Radiopharmaceuticals, Telix-Pharmaceuticals, and ABX Radiopharmaceuticals. LMU is supported by the Clinician Scientist Program of the LMU Munich. W.P. Fendler reports fees from SOFIE Bioscience (research funding), Janssen (consultant, speaker), Calyx (consultant, image review), Bayer (consultant, speaker, research funding), Novartis (speaker, consultant), Telix (speaker), GE Healthcare (speaker), Eczacıbaşı Monrol (speaker), Abx (speaker), Amgen (speaker), and Urotrials (speaker) outside of the submitted work. S. Wegen, U. Nestle, C. Zamboglou, S.K.B. Spohn, N.H. Nicolay, S.A. Koerber, C. La Fougère, E. Fokas, C. Kobe, C. Eze, A.-L. Grosu, A. Holzgreve, R. Werner and N.-S. Schmidt-Hegemann declare that they have no competing interests.
Figures

References
-
- Nestle U, Schimek-Jasch T, Kremp S, Schaefer-Schuler A, Mix M, Kusters A et al (2020) Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): a multicentre, open-label, randomised, controlled trial. Lancet Oncol 21:581–592. 10.1016/S1470-2045(20)30013-9 - DOI - PubMed
-
- Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P et al (2020) Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395:1208–1216. 10.1016/S0140-6736(20)30314-7 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

