Overexpression of long noncoding RNA DUXAP8 inhibits ER-phagy through activating AKT/mTOR signaling and contributes to preeclampsia
- PMID: 39120751
- PMCID: PMC11335266
- DOI: 10.1007/s00018-024-05385-y
Overexpression of long noncoding RNA DUXAP8 inhibits ER-phagy through activating AKT/mTOR signaling and contributes to preeclampsia
Abstract
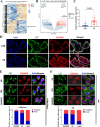
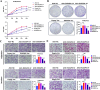
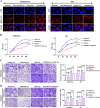
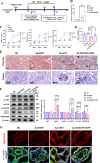
Preeclampsia (PE) is a life-threatening pregnancy-specific complication with controversial mechanisms and no effective treatment except delivery is available. Currently, increasing researchers suggested that PE shares pathophysiologic features with protein misfolding/aggregation disorders, such as Alzheimer disease (AD). Evidences have proposed defective autophagy as a potential source of protein aggregation in PE. Endoplasmic reticulum-selective autophagy (ER-phagy) plays a critical role in clearing misfolded proteins and maintaining ER homeostasis. However, its roles in the molecular pathology of PE remain unclear. We found that lncRNA DUXAP8 was upregulated in preeclamptic placentae and significantly correlated with clinical indicators. DUXAP8 specifically binds to PCBP2 and inhibits its ubiquitination-mediated degradation, and decreased levels of PCBP2 reversed the activation effect of DUXAP8 overexpression on AKT/mTOR signaling pathway. Function experiments showed that DUXAP8 overexpression inhibited trophoblastic proliferation, migration, and invasion of HTR-8/SVneo and JAR cells. Moreover, pathological accumulation of swollen and lytic ER (endoplasmic reticulum) was observed in DUXAP8-overexpressed HTR8/SVneo cells and PE placental villus trophoblast cells, which suggesting that ER clearance ability is impaired. Further studies found that DUXAP8 overexpression impaired ER-phagy and caused protein aggregation medicated by reduced FAM134B and LC3II expression (key proteins involved in ER-phagy) via activating AKT/mTOR signaling pathway. The increased level of FAM134B significantly reversed the inhibitory effect of DUXAP8 overexpression on the proliferation, migration, and invasion of trophoblasts. In vivo, DUXAP8 overexpression through tail vein injection of adenovirus induced PE-like phenotypes in pregnant rats accompanied with activated AKT/mTOR signaling, decreased expression of FAM134B and LC3-II proteins and increased protein aggregation in placental tissues. Our study reveals the important role of lncRNA DUXAP8 in regulating trophoblast biological behaviors through FAM134B-mediated ER-phagy, providing a new theoretical basis for understanding the pathogenesis of PE.
Keywords: DUXAP8; ER-phagy; FAM134B; Preeclampsia; Trophoblast cells.
© 2024. The Author(s).
Conflict of interest statement
None of the authors have any conflicts of interest to declare.
Figures



References
-
- Rana S, Lemoine E, Granger JP, Karumanchi SA (2019) Preeclampsia: Pathophysiology, Challenges, and Perspectives Circ Res, 2019. 124(7): pp. 1094–1112.10.1161/circresaha.118.313276 - PubMed
-
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S et al (2018) Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice Hypertension, 2018. 72(1): pp. 24–43.10.1161/hypertensionaha.117.10803 - PubMed
-
- Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ (2016) Pre-eclampsia The Lancet, 2016. 387(10022): pp. 999–1011.10.1016/S0140-6736(15)00070-7 - PubMed
-
- Hitti J, Sienas L, Walker S, Benedetti TJ, Easterling T (2018) Contribution of hypertension to severe maternal morbidity Am J Obstet Gynecol, 2018. 219(4): p. 40510.1016/j.ajog.2018.07.002 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

