Validity of Patient-Reported Outcome Measures in Evaluating Nerve Damage Following Chemotherapy
- PMID: 39120903
- PMCID: PMC11316238
- DOI: 10.1001/jamanetworkopen.2024.24139
Validity of Patient-Reported Outcome Measures in Evaluating Nerve Damage Following Chemotherapy
Abstract
Importance: Chemotherapy-induced peripheral neuropathy (CIPN) is a substantial adverse effect of anticancer treatments. As such, the assessment of CIPN remains critically important in both research and clinic settings.
Objective: To compare the validity of various patient-reported outcome measures (PROMs) with neurophysiological and sensory functional measures as the optimal method of CIPN assessment.
Design, setting, and participants: This cohort study evaluated participants treated with neurotoxic chemotherapy across 2 cohorts using a dual-study design. Participants commencing treatment were assessed prospectively at beginning of neurotoxic treatment, midtreatment, and at the end of treatment. Participants who completed treatment up to 5 years prior were assessed cross-sectionally and completed a single assessment time point. Participants were recruited from oncology centers in Australia from August 2015 to November 2022. Data analysis occurred from February to November 2023.
Exposures: Neurotoxic cancer treatment including taxanes, platinums, vinca-alkaloids, proteasome inhibitors, and thalidomide.
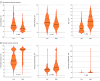
Main outcomes and measures: CIPN was assessed via PROMs (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire [EORTC-CIPN20], Functional Assessment of Cancer Therapy/Gynecological Cancer Group Neurotoxicity Questionnaire (FACT/GOG-Ntx), and the patient-reported outcomes version of the Common Terminology Criteria for Adverse Events [PRO-CTCAE]), neurological and neurophysiological assessment (Total Neuropathy Score and sural and tibial compound nerve amplitudes), and sensory measures (Grating orientation, Von Frey monofilament, and 2-point discrimination tasks). Core measurement properties of CIPN outcome measures were evaluated. Convergent and known-groups validity was assessed cross-sectionally following treatment completion, and responsiveness was evaluated prospectively during treatment. Neurological, neurophysiological, and sensory outcome measure scores were compared between those who reported high and low levels of CIPN symptoms using linear regressions.
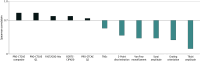
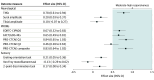
Results: A total of 1033 participants (median [IQR] age, 61 [50-59] years; 676 female [65.4%]) were recruited to this study, incorporating 1623 assessments. PROMs demonstrated best ability to accurately assess CIPN (convergent validity), especially the PRO-CTCAE composite score (r = 0.85; P < .001) and EORTC-CIPN20 (r = 0.79; P < .001). PROMS also demonstrated the best ability to discriminate between CIPN severity (known-groups validity) and to detect changes at onset of CIPN development (responsiveness), especially for EORTC-CIPN20 (d = 0.67; 95% CI, 0.52-0.83), FACT/GOG-Ntx (d = 0.65; 95% CI, 0.49-0.81) and the PRO-CTCAE (d = 0.83; 95% CI, 0.64-1.02). Other measures did not achieve threshold for convergent validity (α < 0.7). Neurophysiological and sensory measures did not demonstrate acceptable responsiveness. In regression models, neurological, neurophysiological, and sensory outcome measures were significantly impaired in participants who reported high levels of CIPN symptoms compared with those who reported low levels of CIPN symptoms.
Conclusions and relevance: In this cohort study of 1033 cancer patients, PROMs were the only measures to satisfy all 3 core measurement property criteria (convergent validity, known-groups validity, and responsiveness). These findings suggest that adoption of PROMs in clinical practice can equip clinicians with valuable information in assessing CIPN morbidity.
Conflict of interest statement
Figures
References
-
- Hershman DL, Weimer LH, Wang A, et al. . Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125(3):767-774. doi:10.1007/s10549-010-1278-0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

