Decreased sirtuin 4 levels promote cellular proliferation and invasion in papillary thyroid carcinoma
- PMID: 39121020
- PMCID: PMC11466257
- DOI: 10.1530/ETJ-24-0079
Decreased sirtuin 4 levels promote cellular proliferation and invasion in papillary thyroid carcinoma
Abstract
Objective: This study examined the effect of sirtuin 4 (SIRT4), a NAD+-dependent deacetylase, on the proliferation and progression of papillary thyroid carcinoma (PTC).
Methods: Data from The Cancer Genome Atlas (TCGA) were analyzed to identify SIRT4 expression in thyroid cancer. Subsequently, the correlation between SIRT4 expression and clinical characteristics was examined in 205 PTC tissue samples. In vitro assays using three human thyroid cancer cell lines (B-CPAP, TPC-1, and SNU-790) were conducted to assess the effects of regulated SIRT4 expression on cell growth, apoptosis, invasion, and migration. Furthermore, in vivo experiments were performed in a xenograft mouse model.
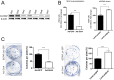
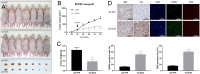
Results: Gene Expression Omnibus (GEO) and TCGA data indicated that SIRT4 expression is lower in thyroid cancer and SIRT4 downregulation is associated with poor overall survival. In PTC tissues, positive SIRT4 expression was associated with decreased extracapsular extension. In in vitro experiments using three human thyroid cancer cell lines, overexpression of SIRT4 decreased cell survival, clonogenic potential, and invasion and migratory capabilities, as well as inducing apoptosis and increasing reactive oxygen species levels. SIRT4 overexpression upregulated E-cadherin and downregulated N-cadherin, suggesting its potential involvement in the regulation of epithelial-mesenchymal transition. These findings were confirmed in vivo using a xenograft mouse model.
Conclusion: This study provides novel insight into the potential contribution of SIRT4 to the regulation of the pathological progression of PTC. The data suggest that SIRT4 plays a tumor-suppressive role in PTC by inhibiting growth, survival, and invasive potential. Future research should investigate the molecular mechanisms underlying these effects of SIRT4.
Keywords: SIRT4 protein; epithelial–mesenchymal transition; papillary thyroid cancer; reactive oxygen species; thyroid cancer.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Agate L Lorusso L & Elisei R. New and old knowledge on differentiated thyroid cancer epidemiology and risk factors. Journal of Endocrinological Investigation 201235(Supplement) 3–9. - PubMed
-
- Hundahl SA Fleming ID Fremgen AM & Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 (see comments). Cancer 1998832638–2648. (https://doi.org/10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr...) - PubMed
-
- Ito Y Kudo T Kobayashi K Miya A Ichihara K & Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World Journal of Surgery 2012361274–1278. (10.1007/s00268-012-1423-5) - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

