Immune cell activity during anti-TNF treatment in patients with psoriasis and psoriatic arthritis
- PMID: 39121030
- PMCID: PMC11557139
- DOI: 10.1093/cei/uxae070
Immune cell activity during anti-TNF treatment in patients with psoriasis and psoriatic arthritis
Abstract
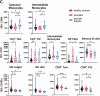
Psoriasis is a chronic, inflammatory skin disease characterized by a dysregulated immune response and systemic inflammation. Up to one-third of patients with psoriasis have psoriatic arthritis (PsA). Targeted treatment with antibodies neutralizing tumor necrosis factor can ameliorate both diseases. We here explored the impact of long-term infliximab treatment on the composition and activity status of circulating immune cells involved in chronic skin and joint inflammation. Immune cells were analyzed by multicolor flow cytometry. We measured markers of immune activation in peripheral blood mononuclear cell populations in 24 infliximab-treated patients with psoriasis/PsA compared to 32 healthy controls. We observed a significant decrease in the frequency of both peripheral natural killer (NK) cells and their subset CD56dimCD16+ NK cells in PsA compared to healthy controls and patients with psoriasis. The latter had a strong-positive correlation with psoriasis area severity index (PASI) in these patients, while CD56brightCD16- NK cells were negatively correlated with PASI. In addition, we observed an upregulation of CD69+ intermediate CD14+CD16+ and CD69+ classical CD14+CD16- monocytes in PsA and increased activity of CD38+ intermediate CD14+CD16+ monocytes in patients with psoriasis. Compared to healthy controls, psoriasis patients demonstrated shifts of the three B-cell subsets with a decrease in transitional CD27-CD38high B cells. Our exploratory study indicates a preserved pathophysiological process including continuous systemic inflammation despite clinical stability of the patients treated with infliximab.
Keywords: B cells; flow cytometry; infliximab; psoriasis; psoriatic arthritis; systemic inflammation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J.. Psoriasis. Lancet 2021, 397, 1301–15. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

