Exposure to Lead in Drinking Water Causes Cognitive Impairment via an Alzheimer's Disease Gene-Dependent Mechanism in Adult Mice
- PMID: 39121129
- PMCID: PMC11616619
- DOI: 10.3233/JAD-240640
Exposure to Lead in Drinking Water Causes Cognitive Impairment via an Alzheimer's Disease Gene-Dependent Mechanism in Adult Mice
Abstract
Background: Exposure to lead (Pb) is a major public health problem that could occur through contaminated soil, air, food, or water, either during the course of everyday life, or while working in hazardous occupations. Although Pb has long been known as a neurodevelopmental toxicant in children, a recent and growing body of epidemiological research indicates that cumulative, low-level Pb exposure likely drives age-related neurologic dysfunction in adults. Environmental Pb exposure in adulthood has been linked to risk of late-onset Alzheimer's disease (AD) and dementia.
Objective: Although the biological mechanism underlying this link is unknown, it has been proposed that Pb exposure may increase the risk of AD via altering the expression of AD-related genes and, possibly, by activating the molecular pathways underlying AD-related pathology.
Methods: We investigated Pb exposure using a line of genetically modified mice with AD-causing knock-in mutations in the amyloid precursor protein and presenilin 1 (APPΔNL/ΔNL x PS1P264L/P264L) that had been crossed with Leprdb/db mice to impart vulnerability to vascular pathology.
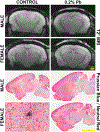
Results: Our data show that although Pb exposure in adult mice impairs cognitive function, this effect is not related to either an increase in amyloid pathology or to changes in the expression of common AD-related genes. Pb exposure also caused a significant increase in blood pressure, a well known effect of Pb. Interestingly, although the increase in blood pressure was unrelated to genotype, only mice that carried AD-related mutations developed cognitive dysfunction, in spite of showing no significant change in cerebrovascular pathology.
Conclusions: These results raise the possibility that the increased risk of dementia associated with Pb exposure in adults may be tied to its subsequent interaction with either pre-existing or developing AD-related neuropathology.
Keywords: Aging; Alzheimer’s disease; amyloid; amyloid precursor protein; hypertension; presenilin 1; vascular contributions to cognitive impairment and dementia.
Conflict of interest statement
CONFLICTS OF INTEREST
M. Paul Murphy is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review. The remaining authors have no conflicts of interest to report.
Figures


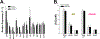

References
-
- Lu K, Liu T, Wu X, et al. Association between serum iron, blood lead, cadmium, mercury, selenium, manganese and low cognitive performance in old adults from National Health and Nutrition Examination Survey (NHANES): a cross-sectional study. Br J Nutr 2023; 130: 1743–1753. - PubMed
-
- Li K, Wu J, Mei Y, et al. Metallomics analysis of metal exposure and cognitive function in older adults: A combined epidemiological and bioinformatics study. Chemosphere 2023; 341: 140049. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

