Plasma myo-inositol elevation in heart failure: clinical implications and prognostic significance. Results from the BElgian and CAnadian MEtabolomics in HFpEF (BECAME-HF) research project
- PMID: 39121579
- PMCID: PMC11363489
- DOI: 10.1016/j.ebiom.2024.105264
Plasma myo-inositol elevation in heart failure: clinical implications and prognostic significance. Results from the BElgian and CAnadian MEtabolomics in HFpEF (BECAME-HF) research project
Abstract
Background: The metabolic environment plays a crucial role in the development of heart failure (HF). Our prior research demonstrated that myo-inositol, a metabolite transported by the sodium-myo-inositol co-transporter 1 (SMIT-1), can induce oxidative stress and may be detrimental to heart function. However, plasmatic myo-inositol concentration has not been comprehensively assessed in large cohorts of patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF).
Methods: Plasmatic myo-inositol levels were measured using mass spectrometry and correlated with clinical characteristics in no HF subjects and patients with HFrEF and HFpEF from Belgian (male, no HF, 53%; HFrEF, 84% and HFpEF, 40%) and Canadian cohorts (male, no HF, 51%; HFrEF, 92% and HFpEF, 62%).
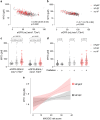
Findings: Myo-inositol levels were significantly elevated in patients with HF, with a more pronounced increase observed in the HFpEF population of both cohorts. After adjusting for age, sex, body mass index, hypertension, diabetes, and atrial fibrillation, we observed that both HFpEF status and impaired kidney function were associated with elevated plasma myo-inositol. Unlike HFrEF, abnormally high myo-inositol (≥69.8 μM) was linked to unfavourable clinical outcomes (hazard ratio, 1.62; 95% confidence interval, [1.05-2.5]) in patients with HFpEF. These elevated levels were correlated with NTproBNP, troponin, and cardiac fibrosis in this subset of patients.
Interpretation: Myo-inositol is a metabolite elevated in patients with HF and strongly correlated to kidney failure. In patients with HFpEF, high myo-inositol levels predict poor clinical outcomes and are linked to markers of cardiac adverse remodelling. This suggests that myo-inositol and its transporter SMIT1 may have a role in the pathophysiology of HFpEF.
Funding: BECAME-HF was supported by Collaborative Bilateral Research Program Québec - Wallonie-Brussels Federation.
Keywords: HFpEF; Heart failure; Kidney; Metabolites; Myo-inositol; Prognosis.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Jean-Claude Tardif reports grants from Amarin, AstraZeneca, Ceapro, DalCor Pharmaceuticals, Esperion, Ionis, Merck, Novartis, Pfizer and RegenXBio; honoraria from AstraZeneca, DalCor Pharmaceuticals, HLS Pharmaceuticals, Pendopharm and Pfizer; minor equity interest in DalCor Pharmaceuticals; and authorship of patents on pharmacogenomics-guided CETP inhibition and use of colchicine after myocardial infarction. No other conflict of interest was declared for the present study.
Figures



References
-
- Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. - PubMed
-
- Solomon S.D., McMurray J.J.V., Claggett B., et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–1098. - PubMed
-
- Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

