A glia-enriched stem cell 3D model of the human brain mimics the glial-immune neurodegenerative phenotypes of multiple sclerosis
- PMID: 39121861
- PMCID: PMC11384947
- DOI: 10.1016/j.xcrm.2024.101680
A glia-enriched stem cell 3D model of the human brain mimics the glial-immune neurodegenerative phenotypes of multiple sclerosis
Abstract
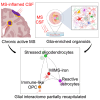
The role of central nervous system (CNS) glia in sustaining self-autonomous inflammation and driving clinical progression in multiple sclerosis (MS) is gaining scientific interest. We applied a single transcription factor (SOX10)-based protocol to accelerate oligodendrocyte differentiation from human induced pluripotent stem cell (hiPSC)-derived neural precursor cells, generating self-organizing forebrain organoids. These organoids include neurons, astrocytes, oligodendroglia, and hiPSC-derived microglia to achieve immunocompetence. Over 8 weeks, organoids reproducibly generated mature CNS cell types, exhibiting single-cell transcriptional profiles similar to the adult human brain. Exposed to inflamed cerebrospinal fluid (CSF) from patients with MS, organoids properly mimic macroglia-microglia neurodegenerative phenotypes and intercellular communication seen in chronic active MS. Oligodendrocyte vulnerability emerged by day 6 post-MS-CSF exposure, with nearly 50% reduction. Temporally resolved organoid data support and expand on the role of soluble CSF mediators in sustaining downstream events leading to oligodendrocyte death and inflammatory neurodegeneration. Such findings support the implementation of this organoid model for drug screening to halt inflammatory neurodegeneration.
Keywords: SOX10; brain organoids; glia-microglia axis; hiPSC; multiple sclerosis; neuroinflammation; oligodendrocytes; single-cell genomics.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.S.R.: research funding from Abata Therapeutics and Sanofi-Genzyme. P.A.C.: research funding from Genentech and previously Principia; consulting honoraria for serving on SABs for NervGen, Idorsia, Biogen, Vaccitech, and Lilly. L.S.: original organoid model(6) is under a patent by Johns Hopkins University, which is licensed to AxoSim, New Orleans, US; she consults for AxoSim. M.A.: consultancy fees from GSK, Sanofi, Biogen, Immunic Therapeutics, and Abata Therapeutics.
Figures




Update of
-
Glia-enriched stem-cell 3D model of the human brain mimics the glial-immune neurodegenerative phenotypes of multiple sclerosis.bioRxiv [Preprint]. 2024 Jun 24:2024.06.20.597748. doi: 10.1101/2024.06.20.597748. bioRxiv. 2024. Update in: Cell Rep Med. 2024 Aug 20;5(8):101680. doi: 10.1016/j.xcrm.2024.101680. PMID: 39372788 Free PMC article. Updated. Preprint.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

