The enzymatic oxygen sensor cysteamine dioxygenase binds its protein substrates through their N-termini
- PMID: 39122008
- PMCID: PMC11406360
- DOI: 10.1016/j.jbc.2024.107653
The enzymatic oxygen sensor cysteamine dioxygenase binds its protein substrates through their N-termini
Abstract
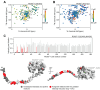
The non-heme iron-dependent dioxygenase 2-aminoethanethiol (aka cysteamine) dioxygenase (ADO) has recently been identified as an enzymatic oxygen sensor that coordinates cellular changes to hypoxia by regulating the stability of proteins bearing an N-terminal cysteine (Nt-cys) through the N-degron pathway. It catalyzes O2-dependent Nt-cys sulfinylation, which promotes proteasomal degradation of the target. Only a few ADO substrates have been verified, including regulators of G-protein signaling (RGS) 4 and 5, and the proinflammatory cytokine interleukin-32, all of which exhibit cell and/or tissue specific expression patterns. ADO, in contrast, is ubiquitously expressed, suggesting it can regulate the stability of additional Nt-cys proteins in an O2-dependent manner. However, the role of individual chemical groups, active site metal, amino acid composition, and globular structure on protein substrate association remains elusive. To help identify new targets and examine the underlying biochemistry of the system, we conducted a series of biophysical experiments to investigate the binding requirements of established ADO substrates RGS5 and interleukin-32. We demonstrate, using surface plasmon response and enzyme assays, that a free, unmodified Nt-thiol and Nt-amine are vital for substrate engagement through active site metal coordination, with residues next to Nt-cys moderately impacting association and catalytic efficiency. Additionally, we show, through 1H-15N heteronuclear single quantum coherence nuclear magnetic resonance titrations, that the globular portion of RGS5 has limited impact on ADO association, with interactions restricted to the N-terminus. This work establishes key features involved in ADO substrate binding, which will help identify new protein targets and, subsequently, elucidate its role in hypoxic adaptation.
Keywords: ADO; N-degron pathway; enzyme kinetics; hypoxia; nuclear magnetic resonance; oxygen-sensing; posttranslational modification; protein degradation; surface plasmon resonance.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Similar articles
-
Characterization of the nonheme iron center of cysteamine dioxygenase and its interaction with substrates.J Biol Chem. 2020 Aug 14;295(33):11789-11802. doi: 10.1074/jbc.RA120.013915. Epub 2020 Jun 28. J Biol Chem. 2020. PMID: 32601061 Free PMC article.
-
Differences in the Second Coordination Sphere Tailor the Substrate Specificity and Reactivity of Thiol Dioxygenases.Acc Chem Res. 2022 Sep 6;55(17):2480-2490. doi: 10.1021/acs.accounts.2c00359. Epub 2022 Aug 22. Acc Chem Res. 2022. PMID: 35994511 Free PMC article.
-
Crystal structure of human cysteamine dioxygenase provides a structural rationale for its function as an oxygen sensor.J Biol Chem. 2021 Oct;297(4):101176. doi: 10.1016/j.jbc.2021.101176. Epub 2021 Sep 8. J Biol Chem. 2021. PMID: 34508780 Free PMC article.
-
Emerging roles for thiol dioxygenases as oxygen sensors.FEBS J. 2022 Sep;289(18):5426-5439. doi: 10.1111/febs.16147. Epub 2021 Aug 27. FEBS J. 2022. PMID: 34346181 Review.
-
Understanding human thiol dioxygenase enzymes: structure to function, and biology to pathology.Int J Exp Pathol. 2017 Apr;98(2):52-66. doi: 10.1111/iep.12222. Epub 2017 Apr 24. Int J Exp Pathol. 2017. PMID: 28439920 Free PMC article. Review.
Cited by
-
An mRNA-display derived cyclic peptide scaffold reveals the substrate binding interactions of an N-terminal cysteine oxidase.Nat Commun. 2025 May 22;16(1):4761. doi: 10.1038/s41467-025-59960-3. Nat Commun. 2025. PMID: 40404614 Free PMC article.
References
-
- Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. - PubMed
-
- Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. - PubMed
-
- Fiorini G., Schofield C.J. Biochemistry of the hypoxia-inducible factor hydroxylases. Curr. Opin. Chem. Biol. 2024;79 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

