A synthetic peptide mimic kills Candida albicans and synergistically prevents infection
- PMID: 39122699
- PMCID: PMC11315985
- DOI: 10.1038/s41467-024-50491-x
A synthetic peptide mimic kills Candida albicans and synergistically prevents infection
Abstract
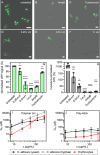
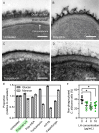
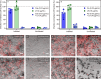
More than two million people worldwide are affected by life-threatening, invasive fungal infections annually. Candida species are the most common cause of nosocomial, invasive fungal infections and are associated with mortality rates above 40%. Despite the increasing incidence of drug-resistance, the development of novel antifungal formulations has been limited. Here we investigate the antifungal mode of action and therapeutic potential of positively charged, synthetic peptide mimics to combat Candida albicans infections. Our data indicates that these synthetic polymers cause endoplasmic reticulum stress and affect protein glycosylation, a mode of action distinct from currently approved antifungal drugs. The most promising polymer composition damaged the mannan layer of the cell wall, with additional membrane-disrupting activity. The synergistic combination of the polymer with caspofungin prevented infection of human epithelial cells in vitro, improved fungal clearance by human macrophages, and significantly increased host survival in a Galleria mellonella model of systemic candidiasis. Additionally, prolonged exposure of C. albicans to the synergistic combination of polymer and caspofungin did not lead to the evolution of tolerant strains in vitro. Together, this work highlights the enormous potential of these synthetic peptide mimics to be used as novel antifungal formulations as well as adjunctive antifungal therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 446404928/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 210879364/Deutsche Forschungsgemeinschaft (German Research Foundation)
- QU116/9-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 434385622 / GR 5617/1-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 210879364/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Medical

