The temporal dynamics of lncRNA Firre-mediated epigenetic and transcriptional regulation
- PMID: 39122712
- PMCID: PMC11316132
- DOI: 10.1038/s41467-024-50402-0
The temporal dynamics of lncRNA Firre-mediated epigenetic and transcriptional regulation
Abstract
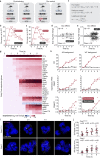
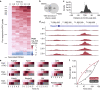
Numerous studies have now demonstrated that lncRNAs can influence gene expression programs leading to cell and organismal phenotypes. Typically, lncRNA perturbations and concomitant changes in gene expression are measured on the timescale of many hours to days. Thus, we currently lack a temporally grounded understanding of the primary, secondary, and tertiary relationships of lncRNA-mediated transcriptional and epigenetic regulation-a prerequisite to elucidating lncRNA mechanisms. To begin to address when and where a lncRNA regulates gene expression, we genetically engineer cell lines to temporally induce the lncRNA Firre. Using this approach, we are able to monitor lncRNA transcriptional regulatory events from 15 min to four days. We observe that upon induction, Firre RNA regulates epigenetic and transcriptional states in trans within 30 min. These early regulatory events result in much larger transcriptional changes after 12 h, well before current studies monitor lncRNA regulation. Moreover, Firre-mediated gene expression changes are epigenetically remembered for days. Overall, this study suggests that lncRNAs can rapidly regulate gene expression by establishing persistent epigenetic and transcriptional states.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

