Clinical outcomes and ctDNA correlates for CAPOX BETR: a phase II trial of capecitabine, oxaliplatin, bevacizumab, trastuzumab in previously untreated advanced HER2+ gastroesophageal adenocarcinoma
- PMID: 39122726
- PMCID: PMC11316091
- DOI: 10.1038/s41467-024-51271-3
Clinical outcomes and ctDNA correlates for CAPOX BETR: a phase II trial of capecitabine, oxaliplatin, bevacizumab, trastuzumab in previously untreated advanced HER2+ gastroesophageal adenocarcinoma
Abstract
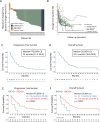
Preclinical studies suggest that simultaneous HER2/VEGF blockade may have cooperative effects in gastroesophageal adenocarcinomas. In a single-arm investigator initiated clinical trial for patients with untreated advanced HER2+ gastroesophageal adenocarcinoma, bevacizumab was added to standard of care capecitabine, oxaliplatin, and trastuzumab in 36 patients (NCT01191697). Primary endpoint was objective response rate and secondary endpoints included safety, duration of response, progression free survival, and overall survival. The study met its primary endpoint with an objective response rate of 81% (95% CI 65-92%). Median progression free and overall survival were 14.0 (95% CI, 11.3-36.4) and 23.2 months (95% CI, 16.6-36.4), respectively. The median duration of response was 14.9 months. The regimen was well tolerated without unexpected or severe toxicities. In post-hoc ctDNA analysis, baseline ctDNA features were prognostic: Higher tumor fraction and alternative MAPK drivers portended worse outcomes. ctDNA at resistance identified oncogenic mutations and these were detectable 2-8 cycles prior to radiographic progression. Capecitabine, oxaliplatin, trastuzumab and bevacizumab shows robust clinical activity in HER2+ gastroesophageal adenocarcinoma. Combination of VEGF inhibitors with chemoimmunotherapy and anti-PD1 regimens is warranted.
© 2024. The Author(s).
Conflict of interest statement
HS receives research funding from AstraZeneca and has consulted for Dewpoint Therapeutics, Inc. and Merck Sharp & Dohme, LLC. RJK reports receiving advisory board/consulting fees from Astellas, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, EMD Serono, Exact Sciences, Grail, Ipsen, Merck, Novartis, Novocure, Phillips, Takeda, Toray and grant support paid to Johns Hopkins University and Baylor University Medical Center from Bristol Myers Squibb and Eli Lilly. TAA receives research funding to his institution from Lilly, Sanofi; receives consulting fees from Eisai and AstraZeneca; stock ownership in Merck. JAC receives consulting fees from Ipsen, Novartis, Pfizer. MY receives research funding from Janssen Pharmaceuticals and fees for peer review services from UpToDate. SJK reports consulting/advisory role for Merck, BMS, Astellas, Eli Lilly, AstraZeneca, Amgen, Daiichi-Sankyo, Novartis, Pfizer, Mersana, and Exact Sciences. SJK reports stock/equity for Turning Point Therapeutics, Nuvalent Therapeutics. JMC receives research funding to his institution from Merus, Roche, Servier, and Bristol Myers Squibb. He receives research support from Merck, AstraZeneca, Esperas Pharma, Bayer, Tesaro, Arcus Biosciences, and Apexigen; he additionally received honoraria for serving on the advisory board of Incyte and Blueprint Medicines. DAR has served as a consultant for Boston Scientific, Celgene, Instylla, Taiho Pharmaceuticals and serves on the scientific advisory board of AxialTx. LLR has received honoraria from PeerView, Medscape, Clinical Care Options, consulting honoraria from Abbvie, Personal Genome Diagnostics, Bristol Myers Squibb, Loxo Oncology at Lilly, Amgen, Meck, AstraZeneca, Sanofi-Genzume, and EMD Serono. LLR is currently an employee of Foundation Medicine, Inc. with stock in Roche Holding AG. AJA has consulted for Anji Pharmaceuticals, Affini-T Therapeutics, Arrakis Therapeutics, AstraZeneca, Boehringer Ingelheim, Oncorus, Inc., Merck & Co., Inc., Mirati Therapeutics, Nimbus Therapeutics, Plexium, Revolution Medicines, Reactive Biosciences, Riva Therapeutics, Servier Pharmaceuticals, Syros Pharmaceuticals, T-knife Therapeutics, Third Rock Ventures, and Ventus Therapeutics. A.J.A. holds equity in Riva Therapeutics. A.J.A. has research funding from Bristol Myers Squibb, Deerfield, Inc., Eli Lilly, Mirati Therapeutics, Novartis, Novo Ventures, Revolution Medicines, and Syros Pharmaceuticals. JAM served on a scientific advisory board for Merck Pharmaceutical. BMW is a consultant for Agenus, Celgene/BMS, EcoR1 Capital, GRAIL, Ipsen, Mirati, Revolution Medicines, and Third Rock Ventures; research support from AstraZeneca, Celgene/BMS, Eli Lilly, Harbinger Health, Novartis, and Revolution Medicines, outside the described work. PCE has served as a consultant and received honoraria from ALX Oncology, Arcus Bioscience, Astellas, Astra-Zeneca, Blueprint Medicines, Bristol-Myers Squibb, Chimeric Therapeutics, Celgene, Coherus, Daiichi-Sankyo, Five Prime, Ideaya, Istari, Legend, Lilly, Loxo, Merck, Novartis, Ono, Servier, Taiho, Takeda, Turning Point Therapeutics, Xencor, Zymeworks. The remaining authors declare no competing interest.
Figures






References
-
- Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet398, 27–40 (2021). 10.1016/S0140-6736(21)00797-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

