Broad-spectrum antibiotics disrupt homeostatic efferocytosis
- PMID: 39122784
- PMCID: PMC7616532
- DOI: 10.1038/s42255-024-01107-7
Broad-spectrum antibiotics disrupt homeostatic efferocytosis
Abstract
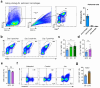
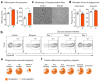
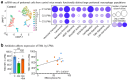
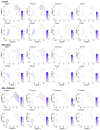
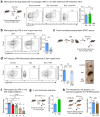
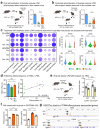
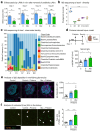
The clearance of apoptotic cells, termed efferocytosis, is essential for tissue homeostasis and prevention of autoimmunity1. Although past studies have elucidated local molecular signals that regulate homeostatic efferocytosis in a tissue2,3, whether signals arising distally also regulate homeostatic efferocytosis remains elusive. Here, we show that large peritoneal macrophage (LPM) display impairs efferocytosis in broad-spectrum antibiotics (ABX)-treated, vancomycin-treated and germ-free mice in vivo, all of which have a depleted gut microbiota. Mechanistically, the microbiota-derived short-chain fatty acid butyrate directly boosts efferocytosis efficiency and capacity in mouse and human macrophages, and rescues ABX-induced LPM efferocytosis defects in vivo. Bulk messenger RNA sequencing of butyrate-treated macrophages in vitro and single-cell messenger RNA sequencing of LPMs isolated from ABX-treated and butyrate-rescued mice reveals regulation of efferocytosis-supportive transcriptional programmes. Specifically, we find that the efferocytosis receptor T cell immunoglobulin and mucin domain containing 4 (TIM-4, Timd4) is downregulated in LPMs of ABX-treated mice but rescued by oral butyrate. We show that TIM-4 is required for the butyrate-induced enhancement of LPM efferocytosis capacity and that LPM efferocytosis is impaired beyond withdrawal of ABX. ABX-treated mice exhibit significantly worse disease in a mouse model of lupus. Our results demonstrate that homeostatic efferocytosis relies on distal metabolic signals and suggest that defective homeostatic efferocytosis may explain the link between ABX use and inflammatory disease4-7.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
J.S.A.P. is a co-founder of Atish Technologies. A.Y.R. is a Scientific Advisory Board member and has equity in Sonoma Biotherapeutics, Santa Ana Bio, RAPT Therapeutics and Vedanta Biosciences. He is a Scientific Executive Board member of Amgen and BioInvent and is a co-inventor or has IP licensed to Takeda that is unrelated to the content of the present study. M.R.M.v.d.B. has received research support and stock options from Seres Therapeutics, and stock options from Notch Therapeutics and Pluto Therapeutics; he has received royalties from Wolters Kluwer; he has consulted, received honorarium from or participated in advisory boards for Seres Therapeutics, Vor Biopharma, Rheos Medicines, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Ceramedix, Lygenesis, Pluto Therapeutics, GlaskoSmithKline, Da Volterra, Thymofox, Garuda, Novartis (Spouse), Synthekine (Spouse), Beigene (Spouse) and Kite (Spouse); he has IP Licensing with Seres Therapeutics and Juno Therapeutics; and he holds a fiduciary role on the Foundation Board of DKMS (a nonprofit organization). Memorial Sloan Kettering has institutional financial interests relative to Seres Therapeutics. The remaining authors declare no competing interests.
Figures




References
-
- Sender R, Milo R. The distribution of cellular turnover in the human body. Nature Medicine. 2021;27:45–48. 2021 27:1. - PubMed
-
- Norgaard M, et al. Use of Penicillin and Other Antibiotics and Risk of Multiple Sclerosis: A Population-based Case-Control Study. American Journal of Epidemiology. 2011;174:945–948. - PubMed
-
- Canova C, et al. Association of Maternal Education, Early Infections, and Antibiotic Use With Celiac Disease: A Population-Based Birth Cohort Study in Northeastern Italy. American Journal of Epidemiology. 2014;180:76–85. - PubMed
MeSH terms
Substances
Grants and funding
- R00 CA237728/CA/NCI NIH HHS/United States
- DP2 GM146337/GM/NIGMS NIH HHS/United States
- MR/X019314/1/RCUK | Medical Research Council (MRC)
- MR/W019264/1/RCUK | Medical Research Council (MRC)
- RM1GM139738/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- P30CA008748/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30 CA008748/CA/NCI NIH HHS/United States
- 225923/Z/22/Z/Wellcome Trust (Wellcome)
- PATH/Burroughs Wellcome Fund (BWF)
- R01 AI148416/AI/NIAID NIH HHS/United States
- 5R00CA237728/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- WT_/Wellcome Trust/United Kingdom
- RM1 GM139738/GM/NIGMS NIH HHS/United States
- R01AI148416/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- MR/X019314/1/MRC_/Medical Research Council/United Kingdom
- MR/W019264/1/MRC_/Medical Research Council/United Kingdom
- 1DP2GM146337/U.S. Department of Health & Human Services | NIH | NIH Office of the Director (OD)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

