Binary classification of copy number alteration profiles in liquid biopsy with potential clinical impact in advanced NSCLC
- PMID: 39122833
- PMCID: PMC11316016
- DOI: 10.1038/s41598-024-68229-6
Binary classification of copy number alteration profiles in liquid biopsy with potential clinical impact in advanced NSCLC
Abstract
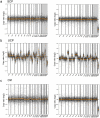
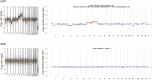
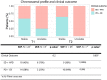
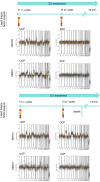
Liquid biopsy has recently emerged as an important tool in clinical practice particularly for lung cancer patients. We retrospectively evaluated cell-free DNA analyses performed at our Institution by next generation sequencing methodology detecting the major classes of genetic alterations. Starting from the graphical representation of chromosomal alterations provided by the analysis software, we developed a support vector machine classifier to automatically classify chromosomal profiles as stable (SCP) or unstable (UCP). High concordance was found between our binary classification and tumor fraction evaluation performed using shallow whole genome sequencing. Among clinical features, UCP patients were more likely to have ≥ 3 metastatic sites and liver metastases. Longitudinal assessment of chromosomal profiles in 33 patients with lung cancer receiving immune checkpoint inhibitors (ICIs) showed that only patients that experienced early death or hyperprogressive disease retained or acquired an UCP within 3 weeks from the beginning of ICIs. UCP was not observed following ICIs among patients that experienced progressive disease or clinical benefit. In conclusion, our binary classification, applied to whole copy number alteration profiles, could be useful for clinical risk stratification during systemic treatment for non-small cell lung cancer patients.
Keywords: Copy number alterations; Immunotherapy; Liquid biopsy; Machine learning; Tumor fraction.
© 2024. The Author(s).
Conflict of interest statement
Dominic Rose is affiliated with Roche Diagnostics Deutschland GmbH, which is the company that provided the assay for this study. The other authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

