Circulating microRNAs as biomarkers for stratifying different phases of liver cancer progression and response to therapy
- PMID: 39122875
- PMCID: PMC11315904
- DOI: 10.1038/s41598-024-69548-4
Circulating microRNAs as biomarkers for stratifying different phases of liver cancer progression and response to therapy
Abstract
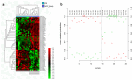
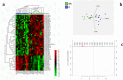
Hepatocellular carcinoma (HCC) is the most common liver cancer and is among the leading causes of cancer-related death worldwide. There is no reliable biomarker for the early diagnosis of HCC. Circulating microRNAs (miRNAs) have attracted attention as potential biomarkers of disease. By small-RNA next-generation sequencing, the analysis of serum miRNAs led to the identification of molecular signatures able to discriminate advanced HCC from early HCC (n = 246); advanced HCC from CIRRHOSIS (n = 299); advanced HCC from HEALTHY (n = 320); HEALTHY from early HCC (n = 343); and HEALTHY from CIRRHOSIS (n = 414). Cirrhotic patients and early HCC patients exhibited similar serum miRNA profiles, yet a small number of miRNAs (n = 57) were able to distinguish these two classes of patients. A second objective of the study was to identify serum miRNAs capable of predicting the response to therapy in patients with advanced HCC. All patients were treated with sorafenib as first-line therapy: 24 were nonresponsive and 24 responsive. Analysis of circulating miRNAs revealed a 54 miRNAs signature able to separate the two subgroups. This study suggested that circulating miRNAs could be useful biomarkers for monitoring patients with liver diseases ranging from cirrhosis to advanced HCC and possibly predicting susceptibility to first-line treatment based on sorafenib.
Keywords: Circulating biomarkers; Diagnostic biormarkers; Early diagnosis; Hepatocellular carcinoma; Next generation sequencing; Predictive biomarkers; Sorafenib; microRNA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Heidelbaugh, J. J. & Bruderly, M. Cirrhosis and chronic liver failure: Part I. Diagnosis and evaluation. Am. Family Physician74, 756–762 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

