Vicia ervilia lectin (VEA) has an antibiofilm effect on both Gram-positive and Gram-negative pathogenic bacteria
- PMID: 39122975
- PMCID: PMC11315768
- DOI: 10.1007/s00203-024-04100-6
Vicia ervilia lectin (VEA) has an antibiofilm effect on both Gram-positive and Gram-negative pathogenic bacteria
Abstract
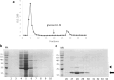
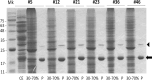
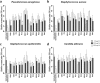
Bacterial growing resistance to antibiotics poses a critical threat to global health. This study investigates, for the first time, the antibiofilm properties of Vicia ervilia agglutinin (VEA) from six different V. ervilia accessions against pathogenic bacteria, and the yeast Candida albicans. In the absence of antimicrobial properties, purified VEA significantly inhibited biofilm formation, both in Gram-positive and Gram-negative bacteria, but not in C. albicans. With an inhibitory concentration ranging from 100 to 500 µg/ml, the VEA antibiofilm activity was more relevant against the Gram-positive bacteria Streptococcus aureus and Staphylococcus epidermidis, whose biofilm was reduced up to 50% by VEA purified from accessions #5 and #36. VEA antibiofilm variability between accessions was observed, likely due to co-purified small molecules rather than differences in VEA protein sequences. In conclusion, VEA seed extracts from the accessions with the highest antibiofilm activity could represent a valid approach for the development of an effective antibiofilm agent.
Keywords: Vicia ervilia; Antibiotic resistance; Antimicrobial; Biofilm; Lectin; Lectin gene.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Ahmed S, Baloch MN, Moin SF, Musa H (2023) Isolation of lectin from Musa acuminata for its antibiofilm potential against Methicillin-resistant Staphylococcus aureus and its synergistic effect with Enterococcus species. Arch Microbiol 205:181. 10.1007/s00203-023-03472-5 10.1007/s00203-023-03472-5 - DOI - PubMed
-
- Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, Stuppner H (2015) Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv 33(8):1582–1614. 10.1016/j.biotechadv.2015.08.001 10.1016/j.biotechadv.2015.08.001 - DOI - PMC - PubMed
-
- Ayouba A, Causse H, Van Damme EJ, Peumans WJ, Bourne Y, Cambillau C, Rougé P (1994) Interactions of plant lectins with the components of the bacterial cell wall peptidoglycan. Biochem Syst Ecol 22(2):153–159. 10.1016/0305-1978(94)90005-110.1016/0305-1978(94)90005-1 - DOI
-
- Azadi S, Azizipour E, Amani AM, Vaez A, Zareshahrabadi Z, Abbaspour A, Firuzyar T, Dortaj H, Kamyab H, Chelliapan S, Mosleh-Shirazi S (2024) Antifungal activity of Fe3O4@SiO2/Schiff-base/Cu(II) magnetic nanoparticles against pathogenic Candida species. Sci Rep 14(1):5855. 10.1038/s41598-024-56512-5 10.1038/s41598-024-56512-5 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- CN00000022/European Union
- CN00000022/European Union
- CN00000022/European Union
- CN00000022/European Union
- CN00000022/European Union
- CN00000022/European Union
- CN00000022/European Union
- Award 2022/Consiglio Nazionale delle Ricerche
- Award 2022/Consiglio Nazionale delle Ricerche
- Award 2022/Consiglio Nazionale delle Ricerche
- Award 2022/Consiglio Nazionale delle Ricerche
- Award 2022/Consiglio Nazionale delle Ricerche
- Award 2022/Consiglio Nazionale delle Ricerche
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

