SGLT2 inhibitors: a novel therapy for cognitive impairment via multifaceted effects on the nervous system
- PMID: 39123214
- PMCID: PMC11312905
- DOI: 10.1186/s40035-024-00431-y
SGLT2 inhibitors: a novel therapy for cognitive impairment via multifaceted effects on the nervous system
Abstract
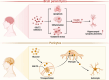
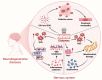
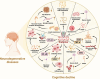
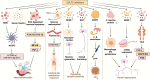
The rising prevalence of diabetes mellitus has casted a spotlight on one of its significant sequelae: cognitive impairment. Sodium-glucose cotransporter-2 (SGLT2) inhibitors, originally developed for diabetes management, are increasingly studied for their cognitive benefits. These benefits may include reduction of oxidative stress and neuroinflammation, decrease of amyloid burdens, enhancement of neuronal plasticity, and improved cerebral glucose utilization. The multifaceted effects and the relatively favorable side-effect profile of SGLT2 inhibitors render them a promising therapeutic candidate for cognitive disorders. Nonetheless, the application of SGLT2 inhibitors for cognitive impairment is not without its limitations, necessitating more comprehensive research to fully determine their therapeutic potential for cognitive treatment. In this review, we discuss the role of SGLT2 in neural function, elucidate the diabetes-cognition nexus, and synthesize current knowledge on the cognitive effects of SGLT2 inhibitors based on animal studies and clinical evidence. Research gaps are proposed to spur further investigation.
Keywords: Cognitive impairment; Diabetes; Neuron; SGLT2 inhibitor; Sodium-glucose cotransporter-2.
© 2024. The Author(s).
Conflict of interest statement
None.
Figures
References
-
- Gudkova AA, Sorokina IB, Iakovlev AA, Guliaeva NV, Gekht AB. Akatinol memantine in patients with vascular cognitive disorders. Zh Nevrol Psikhiatr Im S S Korsakova. 2010;110(12):37–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- No. 82160371/Innovative Research Group Project of the National Natural Science Foundation of China
- No.82100869/Innovative Research Group Project of the National Natural Science Foundation of China
- No.82360162/Innovative Research Group Project of the National Natural Science Foundation of China
- 202201011395/Innovative Research Group Project of the National Natural Science Foundation of China
- 202201011395/Natural Science Foundation of Guangdong Province
LinkOut - more resources
Full Text Sources

