Hyperglycemia-induced Sirt3 downregulation increases microglial aerobic glycolysis and inflammation in diabetic neuropathic pain pathogenesis
- PMID: 39123294
- PMCID: PMC11315676
- DOI: 10.1111/cns.14913
Hyperglycemia-induced Sirt3 downregulation increases microglial aerobic glycolysis and inflammation in diabetic neuropathic pain pathogenesis
Abstract
Background: Hyperglycemia-induced neuroinflammation significantly contributes to diabetic neuropathic pain (DNP), but the underlying mechanisms remain unclear.
Objective: To investigate the role of Sirt3, a mitochondrial deacetylase, in hyperglycemia-induced neuroinflammation and DNP and to explore potential therapeutic interventions.
Method and results: Here, we found that Sirt3 was downregulated in spinal dorsal horn (SDH) of diabetic mice by RNA-sequencing, which was further confirmed at the mRNA and protein level. Sirt3 deficiency exacerbated hyperglycemia-induced neuroinflammation and DNP by enhancing microglial aerobic glycolysis in vivo and in vitro. Overexpression of Sirt3 in microglia alleviated inflammation by reducing aerobic glycolysis. Mechanistically, high-glucose stimulation activated Akt, which phosphorylates and inactivates FoxO1. The inactivation of FoxO1 diminished the transcription of Sirt3. Besides that, we also found that hyperglycemia induced Sirt3 degradation via the mitophagy-lysosomal pathway. Blocking Akt activation by GSK69093 or metformin rescued the degradation of Sirt3 protein and transcription inhibition of Sirt3 mRNA, which substantially diminished hyperglycemia-induced inflammation. Metformin in vivo treatment alleviated neuroinflammation and diabetic neuropathic pain by rescuing hyperglycemia-induced Sirt3 downregulation.
Conclusion: Hyperglycemia induces metabolic reprogramming and inflammatory activation in microglia through the regulation of Sirt3 transcription and degradation. This novel mechanism identifies Sirt3 as a potential drug target for treating DNP.
Keywords: Akt/FoxO1; Sirt3; diabetic neuropathic pain; glycolysis; metformin; neuroinflammation.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no competing interests.
Figures

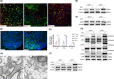



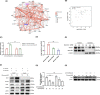
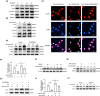
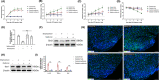
References
-
- Magliano DJ, Boyko EJ. IDF Diabetes Atlas. 2021.
-
- Pesaresi M, Giatti S, Cavaletti G, et al. Sex differences in the manifestation of peripheral diabetic neuropathy in gonadectomized rats: a correlation with the levels of neuroactive steroids in the sciatic nerve. Exp Neurol. 2011;228(2):215‐221. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

