Decoding Clonal Hematopoiesis: Emerging Themes and Novel Mechanistic Insights
- PMID: 39123361
- PMCID: PMC11311828
- DOI: 10.3390/cancers16152634
Decoding Clonal Hematopoiesis: Emerging Themes and Novel Mechanistic Insights
Abstract
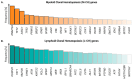
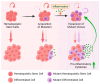
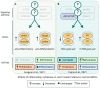
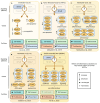
Clonal hematopoiesis (CH), the relative expansion of mutant clones, is derived from hematopoietic stem cells (HSCs) with acquired somatic or cytogenetic alterations that improve cellular fitness. Individuals with CH have a higher risk for hematological and non-hematological diseases, such as cardiovascular disease, and have an overall higher mortality rate. Originally thought to be restricted to a small fraction of elderly people, recent advances in single-cell sequencing and bioinformatics have revealed that CH with multiple expanded mutant clones is universal in the elderly population. Just a few years ago, phylogenetic reconstruction across the human lifespan and novel sensitive sequencing techniques showed that CH can start earlier in life, decades before it was thought possible. These studies also suggest that environmental factors acting through aberrant inflammation might be a common theme promoting clonal expansion and disease progression. However, numerous aspects of this phenomenon remain to be elucidated and the precise mechanisms, context-specific drivers, and pathways of clonal expansion remain to be established. Here, we review our current understanding of the cellular mechanisms driving CH and specifically focus on how pro-inflammatory factors affect normal and mutant HSC fates to promote clonal selection.
Keywords: Asxl1; CHIP; Dnmt3a; Tet2; clonal evolution; clonal hematopoiesis; clonal selection; hematopoietic stem cells; inflammation; self-renewal.
Conflict of interest statement
The authors declare no conflicts of interest, financial or otherwise.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

