Clinical Outcomes and Prognostic Factors in Nonmetastatic Castration-Resistant Prostate Cancer Treated with Androgen Receptor Signaling Inhibitors Therapy
- PMID: 39123387
- PMCID: PMC11312153
- DOI: 10.3390/cancers16152659
Clinical Outcomes and Prognostic Factors in Nonmetastatic Castration-Resistant Prostate Cancer Treated with Androgen Receptor Signaling Inhibitors Therapy
Abstract
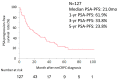
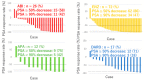
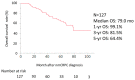
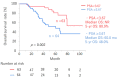
We conducted a retrospective evaluation of the clinical outcomes and prognostic factors in patients with nonmetastatic castration-resistant prostate cancer (nmCRPC) treated with first-line androgen receptor signaling inhibitors (ARSI) in real-world clinical practice in Japan. Between 2012 and 2023, a total of 127 consecutive patients with nmCRPC received ARSI treatment. Overall survival (OS), metastatic-free survival (MFS), and prostate-specific antigen-progression-free survival (PSA-PFS) from ARSI initiation were assessed using the Kaplan-Meier methodology. Clinical factors associated with OS in nmCRPC were analyzed using the Cox proportional hazards model. Among the patients, 72, 26, 12, and 17 received enzalutamide (ENZ), abiraterone (ABI), apalutamide (APA), and darolutamide (DARO) as first-line therapy. The median OS and MFS for all patients were 79.0 and 42.0 months, respectively. Median PSA-PFS was 27.0, 20.0, 10.0, and 14.0 months for patients treated with ENZ, ABI, APA, and DARO, respectively (p = 0.33). Multivariate analysis revealed that a baseline PSA level ≥ 3.67 ng/mL at ARSI initiation was significantly associated with poorer OS (p = 0.002). ARSI demonstrated favorable efficacy in nmCRPC patients. There were no significant differences in clinical outcomes among different types of ARSI therapy for nmCRP. Elevated baseline PSA at ARSI initiation was significantly associated with poorer OS.
Keywords: androgen receptor signaling inhibitors; nonmetastatic castration-resistant prostate cancer; overall survival; prostate-specific antigen; real-world clinical practice.
Conflict of interest statement
R. Fujiwara and T. Yuasa received remuneration for a lecture from Janssen Pharmaceutical K.K. (Tokyo, Japan). T. Yuasa received remuneration for a lecture from Astellas Pharma Inc. (Tokyo, Japan) and Bayer Pharma Japan (Tokyo, Japan). The other authors have declared no conflicts of interest.
Figures


References
-
- Bergengren O., Pekala K.R., Matsoukas K., Fainberg J., Mungovan S.F., Bratt O., Bray F., Brawley O., Luckenbaugh A.N., Mucci L., et al. 2022 update on prostate cancer epidemiology and risk factors—A systematic review. Eur. Urol. 2023;84:191–206. doi: 10.1016/j.eururo.2023.04.021. - DOI - PMC - PubMed
-
- Zumsteg Z.S., Spratt D.E., Romesser P.B., Pei X., Zhang Z., Polkinghorn W., McBride S., Kollmeier M., Yamada Y., Zelefsky M.J. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur. Urol. 2015;67:1009–1016. doi: 10.1016/j.eururo.2014.09.028. - DOI - PMC - PubMed
-
- Boorjian S.A., Thompson R.H., Tollefson M.K., Rangel L.J., Bergstralh E.J., Blute M.L., Karnes R.J. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: The impact of time from surgery to recurrence. Eur. Urol. 2011;59:893–899. doi: 10.1016/j.eururo.2011.02.026. - DOI - PubMed
-
- Aly M., Hashim M., Heeg B., Liwing J., Leval A., Mehra M., Lawson J., Brookman-May S.D., Akre O. Time-to-event outcomes in men with nonmetastatic castrate-resistant prostate cancer-A systematic literature review and pooling of individual participant data. Eur. Urol. Focus. 2019;5:788–798. doi: 10.1016/j.euf.2018.03.010. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

