Omission of Completion Axillary Lymph Node Dissection for Patients with Breast Cancer Treated by Upfront Mastectomy and Sentinel Node Isolated Tumor Cells or Micrometastases
- PMID: 39123393
- PMCID: PMC11312260
- DOI: 10.3390/cancers16152666
Omission of Completion Axillary Lymph Node Dissection for Patients with Breast Cancer Treated by Upfront Mastectomy and Sentinel Node Isolated Tumor Cells or Micrometastases
Abstract
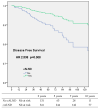
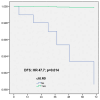
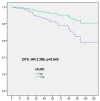
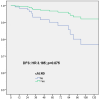
Omission of completion axillary lymph node dissection (cALND) in patients undergoing mastectomy with sentinel node (SN) isolated tumor cells (ITC) or micrometastases is debated due to potential under-treatment, with non-sentinel node (NSN) involvement detected in 7% to 18% of patients. This study evaluated the survival impact of cALND omission in a cohort of breast cancer (BC) patients treated by mastectomy with SN ITC or micrometastases. Among 554 early BC patients (391 pN1mi, 163 ITC), the NSN involvement rate was 13.2% (49/371). With a median follow-up of 66.46 months, multivariate analysis revealed significant associations between cALND omission and overall survival (OS, HR: 2.583, p = 0.043), disease-free survival (DFS, HR: 2.538, p = 0.008), and metastasis-free survival (MFS, HR: 2.756, p = 0.014). For Her2-positive or triple-negative patients, DFS was significantly affected by cALND omission (HR: 38.451, p = 0.030). In ER-positive Her2-negative BC, DFS, OS, recurrence-free survival (RFS), and MFS were significantly associated with cALND omission (DFS HR: 2.358, p = 0.043; OS HR: 3.317; RFS HR: 2.538; MFS HR: 2.756). For 161 patients aged ≤50 years with ER-positive/Her2-negative cancer, OS and breast cancer-specific survival (BCSS) were notably impacted by cALND omission (OS HR: 103.47, p = 0.004; BCSS HR: 50.874, p = 0.035). These findings suggest a potential negative prognostic impact of cALND omission in patients with SN micrometastases or ITC. Further randomized trials are needed.
Keywords: axillary lymph node dissection; early breast cancer; micrometastases; sentinel lymph node.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Krag D.N., Anderson S.J., Julian T.B., Brown A.M., Harlow S.P., Ashikaga T., Weaver D.L., Miller B.J., Jalovec L.M., Frazier T.G., et al. Technical outcomes of sentinel lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. - DOI - PubMed
-
- Ashikaga T., Krag D.N., Land S.R., Julian T.B., Anderson S.J., Brown A.M., Skelly J.M., Harlow S.P., Weaver D.L., Mamounas E.P., et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J. Surg. Oncol. 2010;102:111–118. doi: 10.1002/jso.21535. - DOI - PMC - PubMed
-
- Land S.R., Kopec J.A., Julian T.B., Brown A.M., Anderson S.J., Krag D.N., Christian N.J., Costantino J.P., Wolmark N., Ganz P.A. Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B-32. J. Clin. Oncol. 2010;28:3929–3936. doi: 10.1200/JCO.2010.28.2491. - DOI - PMC - PubMed
-
- Giuliano A.E., Hunt K.K., Ballman K.V., Beitsch P.D., Whitworth P.W., Blumencranz P.W., Leitch A.M., Saha S., McCall L.M., Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. - DOI - PMC - PubMed
-
- Fleissig A., Fallowfield L.J., Langridge C.I., Johnson L., Newcombe R.G., Dixon M., Kissin M., Mansel R.E. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res. Treat. 2006;95:279–293. doi: 10.1007/s10549-005-9025-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

