The Leukemic Isocitrate Dehydrogenase (IDH) 1/2 Mutations Impair Myeloid and Erythroid Cell Differentiation of Primary Human Hematopoietic Stem and Progenitor Cells (HSPCs)
- PMID: 39123404
- PMCID: PMC11312189
- DOI: 10.3390/cancers16152675
The Leukemic Isocitrate Dehydrogenase (IDH) 1/2 Mutations Impair Myeloid and Erythroid Cell Differentiation of Primary Human Hematopoietic Stem and Progenitor Cells (HSPCs)
Abstract
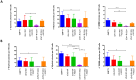
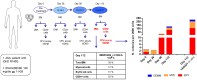
How hematopoietic stem and progenitor cell (HSPC) fate decisions are affected by genetic alterations acquired during AML leukemogenesis is poorly understood and mainly explored in animal models. Here, we study isocitrate dehydrogenase (IDH) gene mutations in the human model of HSPC and discuss the available literature on this topic. IDH1/2 mutations occur in ~20% of AML cases, are recognized among the mutations earliest acquired during leukemogenesis, and are targets of specific inhibitors (ivosidenib and enasidenib, respectively). In order to investigate the direct effects of these mutations on HSPCs, we expressed IDH1-R132H or IDH2-R140Q mutants into human CD34+ healthy donor cells via lentiviral transduction and analyzed the colony-forming unit (CFU) ability. CFU ability was dramatically compromised with a complete trilineage block of differentiation. Strikingly, the block was reversed by specific inhibitors, confirming that it was a specific effect induced by the mutants. In line with this observation, the CD34+ leukemic precursors isolated from a patient with IDH2-mutated AML at baseline and during enasidenib treatment showed progressive and marked improvements in their fitness over time, in terms of CFU ability and propensity to differentiate. They attained clonal trilinear reconstitution of hematopoiesis and complete hematological remission.
Keywords: IDH1/2 mutation; acute myeloid leukemia; human HSPC modeling.
Conflict of interest statement
Maria Paola Martelli declares honoraria/consultancy at the scientific advisory board for Abbvie, Amgen, BMS, Delbert, Janssen, Novartis, Pfizer, and Jazz Pharmaceuticals. The other authors declare no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

