H-1 Parvovirus-Induced Oncolysis and Tumor Microenvironment Immune Modulation in a Novel Heterotypic Spheroid Model of Cutaneous T-Cell Lymphoma
- PMID: 39123440
- PMCID: PMC11311363
- DOI: 10.3390/cancers16152711
H-1 Parvovirus-Induced Oncolysis and Tumor Microenvironment Immune Modulation in a Novel Heterotypic Spheroid Model of Cutaneous T-Cell Lymphoma
Abstract
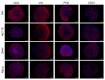
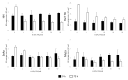
The rat protoparvovirus H-1 (H-1PV) is an oncolytic virus known for its anticancer properties in laboratory models of various human tumors, including non-Hodgkin lymphomas (NHL) of B-cell origin. However, H-1PV therapeutic potential against hematological malignancies of T-cell origin remains underexplored. The aim of the present study was to conduct a pilot preclinical investigation of H-1PV-mediated oncolytic effects in cutaneous T-cell lymphoma (CTCL), a type of NHL that is urgently calling for innovative therapies. We demonstrated H-1PV productive infection and induction of oncolysis in both classically grown CTCL suspension cultures and in a novel, in vivo-relevant, heterotypic spheroid model, but not in healthy donor controls, including peripheral blood mononuclear cells (PBMCs). H-1PV-mediated oncolysis of CTCL cells was not prevented by Bcl-2 overexpression and was accompanied by increased extracellular ATP release. In CTCL spheroid co-cultures with PBMCs, increased spheroid infiltration with immune cells was detected upon co-culture treatment with the virus. In conclusion, our preclinical data show that H-1PV may hold significant potential as an ingenious viroimmunotherapeutic drug candidate against CTCL.
Keywords: cutaneous T-cell lymphoma (CTCL); heterotypic CTCL spheroid; oncolytic H-1 parvovirus; virotherapy.
Conflict of interest statement
M.B. and A.J. declare no conflicts of interest. B.L., J.R. and A.A. are co-inventors in various patents related to anticancer applications of H-1PV. G.U. is the founder and CMO/CSO of CanVirex, Basel, Switzerland.
Figures













References
-
- Olsen E., Vonderheid E., Pimpinelli N., Willemze R., Kim Y., Knobler R., Zackheim H., Duvic M., Estrach T., Lamberg S., et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

