Corrosion Resistance of Coatings Based on Chromium and Aluminum of Titanium Alloy Ti-6Al-4V
- PMID: 39124544
- PMCID: PMC11313918
- DOI: 10.3390/ma17153880
Corrosion Resistance of Coatings Based on Chromium and Aluminum of Titanium Alloy Ti-6Al-4V
Abstract
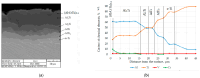
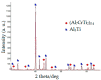
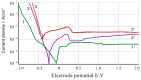
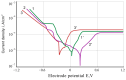
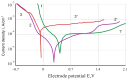
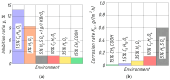
Improvement of wear, corrosion, and heat-resistant properties of coatings to expand the operational capabilities of metals and alloys is an urgent problem for modern enterprises. Diffusion titanium, chromium, and aluminum-based coatings are widely used to solve this challenge. The article aims to obtain the corrosion-electrochemical properties and increase the microhardness of the obtained coatings compared with the initial Ti-6Al-4V alloy. For this purpose, corrosion resistance, massometric tests, and microstructural analysis were applied, considering various aggressive environments (acids, sodium carbonate, and hydrogen peroxide) at different concentrations, treatment temperatures, and saturation times. As a result, corrosion rates, polarization curves, and X-ray microstructures of the uncoated and coated Ti-6Al-4V titanium alloy samples were obtained. Histograms of corrosion inhibition ratio for the chromium-aluminum coatings in various environments were discussed. Overall, the microhardness of the obtained coatings was increased 2.3 times compared with the initial Ti-6Al-4V alloy. The corrosion-resistant chromaluminizing alloy in aqueous solutions of organic acids and hydrogen peroxide was recommended for practical application in conditions of exposure to titanium products.
Keywords: aluminum; chemical-thermal treatment; chromium; corrosion-resistant coating; functional layer; process innovation.
Conflict of interest statement
Author Iryna Smokovych was employed by the company thyssenkrupp Materials Trading GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Chou W.-J., Yu G.-P., Huang J.-H. Corrosion behavior of TiN-coated 304 stainless steel. Corros. Sci. 2001;43:2023–2035. doi: 10.1016/S0010-938X(01)00010-5. - DOI
-
- Bell T. Surface engineering of austenitic stainless steel. Surf. Eng. 2002;18:415–422. doi: 10.1179/026708402225006268. - DOI
-
- Saurabh A., Meghana C.M., Singh P.K., Verma P.C. Titanium-based materials: Synthesis, properties, and applications. Mater. Today Proc. 2022;56:412–419. doi: 10.1016/j.matpr.2022.01.268. - DOI
-
- Medvedovski E., Chinski F.A., Stewart J. Innovative Processing and Manufacturing of Advanced Ceramics and Composites II: Ceramic Transactions. Volume 243. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2014. Thermal diffusion coatings for wear-resistant components for oil and gas industry; pp. 13–29. - DOI
-
- Kandeva M., Zagorski M., Nikolič R., Stojanović B., But A., Botko F., Piteľ J., Vencl A. Friction properties of the heat-treated electroless Ni coatings embedded with c-BN nanoparticles. Coatings. 2022;12:1008. doi: 10.3390/coatings12071008. - DOI
Grants and funding
- APVV-19-0590/Slovak Research and Development Agency
- VEGA 1/0700/24/Ministry of Education, Science, Research and Sport of the Slovak Republic
- KEGA 022TUKE-4/2023/Ministry of Education, Science, Research and Sport of the Slovak Republic
- 09I03-03-V01-00093/EU NextGenerationEU through the Recovery and Resilience Plan for Slovakia
- 09I03-03-V01-00094/EU NextGenerationEU through the Recovery and Resilience Plan for Slovakia
LinkOut - more resources
Full Text Sources
Research Materials

