Clinical Significance and Patterns of Potential Drug-Drug Interactions in Cardiovascular Patients: Focus on Low-Dose Aspirin and Angiotensin-Converting Enzyme Inhibitors
- PMID: 39124556
- PMCID: PMC11313610
- DOI: 10.3390/jcm13154289
Clinical Significance and Patterns of Potential Drug-Drug Interactions in Cardiovascular Patients: Focus on Low-Dose Aspirin and Angiotensin-Converting Enzyme Inhibitors
Abstract
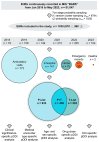
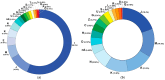
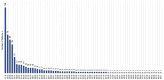
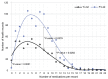
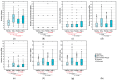
Objective: This study assessed the patterns and clinical significance of potential drug-drug interactions (pDDIs) in patients with diseases of the cardiovascular system. Methods: Electronic health records (EHRs), established in 2018-2023, were selected using the probability serial nested sampling method (n = 1030). Patients were aged 27 to 95 years (65.0% men). Primary diagnosis of COVID-19 was present in 17 EHRs (1.7%). Medscape Drug Interaction Checker was used to characterize pDDIs. The Mann-Whitney U test and chi-square test were used for statistical analysis. Results: Drug numbers per record ranged from 1 to 23 in T-List and from 1 to 20 in P-List. In T-List, 567 drug combinations resulted in 3781 pDDIs. In P-List, 584 drug combinations resulted in 5185 pDDIs. Polypharmacy was detected in 39.0% of records in T-List versus 65.9% in P-List (p-value < 0.05). The rates of serious and monitor-closely pDDIs due to 'aspirin + captopril' combinations were significantly higher in P-List than in T-List (p-value < 0.05). The rates of serious pDDIs due to 'aspirin + enalapril' and 'aspirin + lisinopril' combinations were significantly lower in P-List compared with the corresponding rates in T-List (p-value < 0.05). Serious pDDIs due to administration of aspirin with fosinopril, perindopril, and ramipril were detected less frequently in T-List (p-value < 0.05). Conclusions: Obtained data may suggest better patient adherence to 'aspirin + enalapril' and 'aspirin + lisinopril' combinations, which are potentially superior to the combinations of aspirin with fosinopril, perindopril, and ramipril. An abundance of high-order pDDIs in real-world clinical practice warrants the development of a decision support system aimed at reducing pharmacotherapy-associated risks while integrating patient pharmacokinetic, pharmacodynamic, and pharmacogenetic information.
Keywords: COVID-19; angiotensin-converting enzyme inhibitor; aspirin; cardiovascular disease; polypharmacy; potential drug–drug interaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Anfinogenova N.D., Novikova O.M., Trubacheva I.A., Efimova E.V., Chesalov N.P., Ussov W.Y., Maksimova A.S., Shelkovnikova T.A., Ryumshina N.I., Stepanov V.A., et al. Prescribed versus taken polypharmacy and drug-drug interactions in older cardiovascular patients during the COVID-19 pandemic: Observational cross-sectional analytical study. J. Clin. Med. 2023;12:5061. doi: 10.3390/jcm12155061. - DOI - PMC - PubMed
-
- Dornquast C., Dombrowski M., Zabel M., Willich S.N., Reinhold T. Potential drug-drug interactions in patients with indication for prophylactic implantation of a cardioverter defibrillator: A cross-sectional analysis. BMC Health Serv. Res. 2020;20:271. doi: 10.1186/s12913-020-05131-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

