Beneficial Effects on Exercise Capacity Associated with a Combination of Lactoferrin, Lysozyme, Lactobacillus, Resveratrol, Vitamins, and Oligoelements in Patients with Post-COVID-19 Syndrome: A Single-Center Retrospective Study
- PMID: 39124710
- PMCID: PMC11313403
- DOI: 10.3390/jcm13154444
Beneficial Effects on Exercise Capacity Associated with a Combination of Lactoferrin, Lysozyme, Lactobacillus, Resveratrol, Vitamins, and Oligoelements in Patients with Post-COVID-19 Syndrome: A Single-Center Retrospective Study
Abstract
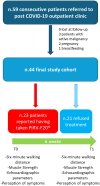
Background/Objectives: Although long-term COVID-19 symptoms are common, little is known about the management of post-COVID-19 condition. The aim of the current report is to evaluate the effects of a combination of lactoferrin, lysozyme, lactobacillus, resveratrol, vitamins, and oligoelements (PIRV-F20®) on the exercise capacity of post-COVID-19 patients. Methods: A retrospective analysis of consecutive patients referred to a specific outpatient clinic dedicated to post-COVID-19 condition from April 2022 to April 2023 was conducted. Subjects of both sexes, aged ≥18 years, with previous COVID-19 in the preceding 12 months, persistent symptoms consistent with post-COVID syndrome, and initial exercise impairment were included. Exclusion criteria were as follows: active cancer, end-stage conditions, severe musculoskeletal conditions, or patients with a history of limited functional capacity, pregnancy, or breastfeeding. Patients who reported having taken PIRV-F20® for at least 6 weeks were compared to patients who refused this treatment. Six-minute walking distance was the primary endpoint. Results: Forty-four patients (56.8% women, aged 49.1 ± 18.1 years) were included in the study. The group of patients who reported having taken PIRV-F20® exhibited a significant improvement of 6MWD (median: +40 m; IQR: 10-65 m, p vs. baseline: 0.02), which was significantly superior (p: 0.01) when compared to the controls (median: +10 m; IQR: -5-30 m). No differences were found with regard to muscular strength, echocardiographic parameters, and perception of symptoms. Conclusions: Post-COVID-19 individuals who reported having taken PIRV-F20® for at least six weeks showed a significant improvement in exercise capacity. This finding should be confirmed in larger, prospective, randomized controlled trials.
Keywords: PIRV-F20®; exercise impairment; natural supplement; post-COVID-19 syndrome.
Conflict of interest statement
A.C., A.M.M., and G.S. received an unrestricted grant from Farmagens Health Care. The authors declare no further conflicts of interest.
Figures

References
-
- Wang Y., Wang Y., Luo W., Huang L., Xiao J., Li F., Qin S., Song X., Wu Y., Zeng Q., et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int. J. Med. Sci. 2020;17:1522–1531. doi: 10.7150/ijms.46695. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

