Recent Advances on Pt-Based Compounds for Theranostic Applications
- PMID: 39124859
- PMCID: PMC11313463
- DOI: 10.3390/molecules29153453
Recent Advances on Pt-Based Compounds for Theranostic Applications
Abstract
Since the discovery of cisplatin's antitumoral activity and its approval as an anticancer drug, significant efforts have been made to enhance its physiological stability and anticancer efficacy and to reduce its side effects. With the rapid development of targeted and personalized therapies, and the promising theranostic approach, platinum drugs have found new opportunities in more sophisticated systems. Theranostic agents combine diagnostic and therapeutic moieties in one scaffold, enabling simultaneous disease monitoring, therapy delivery, response tracking, and treatment efficacy evaluation. In these systems, the platinum core serves as the therapeutic agent, while the functionalized ligand provides diagnostic tools using various imaging techniques. This review aims to highlight the significant role of platinum-based complexes in theranostic applications, and, to the best of our knowledge, this is the first focused contribution on this type of platinum compounds. This review presents a brief introduction to the development of platinum chemotherapeutic drugs, their limitations, and resistance mechanisms. It then describes recent advancements in integrating platinum complexes with diagnostic agents for both tumor treatment and monitoring. The main body is organized into three categories based on imaging techniques: fluorescence, positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI). Finally, this review outlines promising strategies and future perspectives in this evolving field.
Keywords: cancer therapy; molecular imaging; platinum chemotherapy; theranostic; therapy monitoring.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

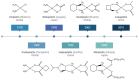



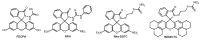


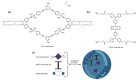
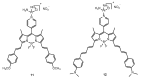















References
-
- Peng K., Liang B.B., Liu W., Mao Z.W. What Blocks More Anticancer Platinum Complexes from Experiment to Clinic: Major Problems and Potential Strategies from Drug Design Perspectives. Coord. Chem. Rev. 2021;449:214210. doi: 10.1016/j.ccr.2021.214210. - DOI
-
- Theiner S., Varbanov H.P., Galanski M., Egger A.E., Berger W., Heffeter P., Keppler B.K. Comparative In Vitro and In Vivo Pharmacological Investigation of Platinum(IV) Complexes as Novel Anticancer Drug Candidates for Oral Application. J. Biol. Inorg. Chem. 2015;20:89–99. doi: 10.1007/s00775-014-1214-6. - DOI - PMC - PubMed
-
- Kim E.S., Tang X.M., Peterson D.R., Kilari D., Chow C.W., Fujimoto J., Kalhor N., Swisher S.G., Stewart D.J., Wistuba I.I., et al. Copper Transporter CTR1 Expression and Tissue Platinum Concentration in Non-Small Cell Lung Cancer. Lung Cancer. 2014;85:88–93. doi: 10.1016/j.lungcan.2014.04.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

